Abstract
Purpose
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive interstitial lung disease characterized by excessive extracellular matrix deposition. No effective treatments are currently available for IPF. High-temperature requirement A3 (HtrA3) suppresses tumor development by antagonizing transforming growth factor β (TGF-β) signaling; however, little is known about the role of HtrA3 in IPF. This study investigated the role of HtrA3 in IPF and underlying mechanisms.
Methods
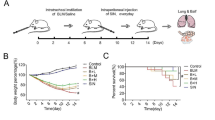
Lung tissues were collected from patients with IPF and mice with bleomycin (BLM)-induced pulmonary fibrosis, and HtrA3 expression was measured in tissue samples. Then, HtrA3 gene knockout mice were treated with BLM to induce pulmonary fibrosis and explore the effects and underlying mechanism of HtrA3 on pulmonary fibrosis.
Results
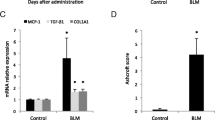
HtrA3 was up-regulated in the lung tissues of patients with IPF and the pulmonary fibrotic mouse model compared to corresponding control groups. HtrA3 knockout decreased pulmonary fibrosis-related protein expression, alleviated the symptoms of pulmonary fibrosis, and inhibited epithelial-mesenchymal transition (EMT) in BLM-induced lung tissue compared with BLM-induced wild-type mice. The TGF-β1/Smad signaling pathway was activated in fibrotic lung tissue, whereas HtrA3 knockout inhibited this pathway.
Conclusion
The expression level of HtrA3 is increased in fibrotic lungs. HtrA3 knockout alleviates the symptoms of pulmonary fibrosis probably via the TGF-β1/Smad signaling pathway. Therefore, HtrA3 inhibition is a potential therapeutic target for pulmonary fibrosis.




Similar content being viewed by others
Data Availability
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
References
Sgalla G, Biffi A, Richeldi L (2016) Idiopathic pulmonary fibrosis: diagnosis, epidemiology and natural history. Respirology 21(3):427–437. https://doi.org/10.1111/resp.12683
Sharif R (2017) Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am J Manag Care 23(11 Suppl):S176–S182
Fernandez Perez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, Yi ES, Ryu JH (2010) Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest 137(1):129–137. https://doi.org/10.1378/chest.09-1002
Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, Patel H, Kreuter M (2021) Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res 22(1):197. https://doi.org/10.1186/s12931-021-01791-z
Lai CC, Wang CY, Lu HM, Chen L, Teng NC, Yan YH, Wang JY, Chang YT, Chao TT, Lin HI, Chen CR, Yu CJ, Wang JD (2012) Idiopathic pulmonary fibrosis in Taiwan - a population-based study. Respir Med 106(11):1566–1574. https://doi.org/10.1016/j.rmed.2012.07.012
Lee HE, Myong JP, Kim HR, Rhee CK, Yoon HK, Koo JW (2016) Incidence and prevalence of idiopathic interstitial pneumonia and idiopathic pulmonary fibrosis in Korea. Int J Tuberc Lung Dis 20(7):978–984. https://doi.org/10.5588/ijtld.16.0003
Kim SW, Myong JP, Yoon HK, Koo JW, Kwon SS, Kim YH (2017) Health care burden and medical resource utilisation of idiopathic pulmonary fibrosis in Korea. Int J Tuberc Lung Dis 21(2):230–235. https://doi.org/10.5588/ijtld.16.0402
Varone F, Sgalla G, Iovene B, Bruni T, Richeldi L (2018) Nintedanib for the treatment of idiopathic pulmonary fibrosis. Expert Opin Pharmacother 19(2):167–175. https://doi.org/10.1080/14656566.2018.1425681
Lancaster LH, de Andrade JA, Zibrak JD, Padilla ML, Albera C, Nathan SD, Wijsenbeek MS, Stauffer JL, Kirchgaessler KU, Costabel U (2017) Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. https://doi.org/10.1183/16000617.0057-2017
Fernandez IE, Eickelberg O (2012) The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9(3):111–116. https://doi.org/10.1513/pats.201203-023AW
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY (2018) New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact 292:76–83. https://doi.org/10.1016/j.cbi.2018.07.008
Fang Y, Tian J, Fan Y, Cao P (2020) Latest progress on the molecular mechanisms of idiopathic pulmonary fibrosis. Mol Biol Rep 47(12):9811–9820. https://doi.org/10.1007/s11033-020-06000-6
Ye Z, Hu Y (2021) TGFbeta1: gentlemanly orchestrator in idiopathic pulmonary fibrosis (Review). Int J Mol Med. https://doi.org/10.3892/ijmm.2021.4965
Wu L, Li X, Li Z, Cheng Y, Wu F, Lv C, Zhang W, Tang W (2021) HtrA serine proteases in cancers: a target of interest for cancer therapy. Biomed Pharmacother 139:111603. https://doi.org/10.1016/j.biopha.2021.111603
Zhao J, Feng M, Liu D, Liu H, Shi M, Zhang J, Qu J (2019) Antagonism between HTRA3 and TGFbeta1 Contributes to Metastasis in Non-Small Cell Lung Cancer. Cancer Res 79(11):2853–2864. https://doi.org/10.1158/0008-5472.CAN-18-2507
Yin Y, Wu M, Nie G, Wang K, Wei J, Zhao M, Chen Q (2013) HtrA3 is negatively correlated with lymph node metastasis in invasive ductal breast cancer. Tumour Biol 34(6):3611–3617. https://doi.org/10.1007/s13277-013-0942-5
Forse CL, Rahimi M, Diamandis EP, Assarzadegan N, Dawson H, Grin A, Kennedy E, O’Connor B, Messenger DE, Riddell RH, Kirsch R, Karagiannis GS (2017) HtrA3 stromal expression is correlated with tumor budding in stage II colorectal cancer. Exp Mol Pathol 103(1):94–100. https://doi.org/10.1016/j.yexmp.2017.07.002
Sauleda J, Nunez B, Sala E, Soriano JB (2018) Idiopathic pulmonary fibrosis: epidemiology, natural history phenotypes. Med Sci (Basel). https://doi.org/10.3390/medsci6040110
Richeldi L, Rubin AS, Avdeev S, Udwadia ZF, Xu ZJ (2015) Idiopathic pulmonary fibrosis in BRIC countries: the cases of Brazil, Russia, India, and China. BMC Med. https://doi.org/10.1186/s12916-015-0495-0
Chien J, Campioni M, Shridhar V, Baldi A (2009) HtrA serine proteases as potential therapeutic targets in cancer. Curr Cancer Drug Targets 9(4):451–468. https://doi.org/10.2174/156800909788486704
Zhao M, Ding JX, Nie GY, Wei J, Li Y, Yin XY, Chen Q (2014) HTRA3 is reduced in ovarian cancers regardless of stage. Cancer Invest 32(9):464–469. https://doi.org/10.3109/07357907.2014.958496
Lv Q, Yang B, Ning C, **e B, Nie G, Chen X, Chen Q (2018) Hypoxia is involved in the reduction of HtrA3 in patients with endometrial hyperplasia and cancer. Biochem Biophys Res Commun 503(4):2918–2923. https://doi.org/10.1016/j.bbrc.2018.08.070
Tzouvelekis A, Gomatou G, Bouros E, Trigidou R, Tzilas V, Bouros D (2019) Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest 156(2):383–391. https://doi.org/10.1016/j.chest.2019.04.114
Rogers TK (2004) Fibroblastic foci in usual interstitial pneumonia. Am J Respir Crit Care Med 169(5):654. https://doi.org/10.1164/ajrccm.169.5.950
Mori Y, Kondoh Y (2021) What parameters can be used to identify early idiopathic pulmonary fibrosis? Respir Investig 59(1):53–65. https://doi.org/10.1016/j.resinv.2020.10.008
Schiller HB, Fernandez IE, Burgstaller G, Schaab C, Scheltema RA, Schwarzmayr T, Strom TM, Eickelberg O, Mann M (2015) Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol 11(7):819. https://doi.org/10.15252/msb.20156123
Deng Z, Fear MW, Suk Choi Y, Wood FM, Allahham A, Mutsaers SE, Prele CM (2020) The extracellular matrix and mechanotransduction in pulmonary fibrosis. Int J Biochem Cell Biol 126:105802. https://doi.org/10.1016/j.biocel.2020.105802
Rosmark O, Ahrman E, Muller C, Elowsson Rendin L, Eriksson L, Malmstrom A, Hallgren O, Larsson-Callerfelt AK, Westergren-Thorsson G, Malmstrom J (2018) Quantifying extracellular matrix turnover in human lung scaffold cultures. Sci Rep 8(1):5409. https://doi.org/10.1038/s41598-018-23702-x
Decaris ML, Gatmaitan M, FlorCruz S, Luo F, Li K, Holmes WE, Hellerstein MK, Turner SM, Emson CL (2014) Proteomic analysis of altered extracellular matrix turnover in bleomycin-induced pulmonary fibrosis. Mol Cell Proteomics 13(7):1741–1752. https://doi.org/10.1074/mcp.M113.037267
Ba YD, Sun JH, Zhao XX (2020) Evogliptin attenuates bleomycin-induced lung fibrosis via inhibiting TGF-beta/Smad signaling in fibroblast. Eur Rev Med Pharmacol Sci 24(20):10790–10798. https://doi.org/10.26355/eurrev_202010_23439
Li A, **ao X, Feng ZL, Chen X, Liu LJ, Lin LG, Lu JJ, Zhang LL (2020) Nagilactone D ameliorates experimental pulmonary fibrosis in vitro and in vivo via modulating TGF-beta/Smad signaling pathway. Toxicol Appl Pharmacol 389:114882. https://doi.org/10.1016/j.taap.2020.114882
Funding
This present study was supported by the Natural Science Foundation of Jiangsu Province (No. BK20190150), the National Natural Science Foundation of China (No.82070059).
Author information
Authors and Affiliations
Contributions
GL: designed the study, analyzed the data and wrote the manuscript. GL, DW and CS: acquired and analyzed data. DW, XCY and WM: collected clinical samples and performed clinical experiments. XSY, CJ and JT: performed animal experiments. JZ: analyzed the data and helped to write the paper. GL and JC: provided the financial support for this work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
The study was approved by the Ethics Committee for the Use of Human Samples of Nan**g Medical University in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All participants gave written informed consent.
Research Involving Human and Animal Participants
All animal experiments were approved by the Institutional Animal Care and Use Committee of Nan**g Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, G., Shen, C., Wei, D. et al. Deficiency of HtrA3 Attenuates Bleomycin-Induced Pulmonary Fibrosis Via TGF-β1/Smad Signaling Pathway. Lung 201, 235–242 (2023). https://doi.org/10.1007/s00408-023-00608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-023-00608-8