Abstract
Labelling medical images is an arduous and costly task that necessitates clinical expertise and large numbers of qualified images. Insufficient samples can lead to underfitting during training and poor performance of supervised learning models. In this study, we aim to develop a SimCLR-based semi-supervised learning framework to classify colorectal neoplasia based on the NICE classification. First, the proposed framework was trained under self-supervised learning using a large unlabelled dataset; subsequently, it was fine-tuned on a limited labelled dataset based on the NICE classification. The model was evaluated on an independent dataset and compared with models based on supervised transfer learning and endoscopists using accuracy, Matthew’s correlation coefficient (MCC), and Cohen’s kappa. Finally, Grad-CAM and t-SNE were applied to visualize the models’ interpretations. A ResNet-backboned SimCLR model (accuracy of 0.908, MCC of 0.862, and Cohen’s kappa of 0.896) outperformed supervised transfer learning-based models (means: 0.803, 0.698, and 0.742) and junior endoscopists (0.816, 0.724, and 0.863), while performing only slightly worse than senior endoscopists (0.916, 0.875, and 0.944). Moreover, t-SNE showed a better clustering of ternary samples through self-supervised learning in SimCLR than through supervised transfer learning. Compared with traditional supervised learning, semi-supervised learning enables deep learning models to achieve improved performance with limited labelled endoscopic images.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impressive ability of deep learning to learn diverse tasks from vast data sources has greatly contributed to prolific advancements in modern computer vision [1]. The generation of big data in scientific fields has coincided with this growth. Through the utilization of vast datasets, computer vision models have acquired a variety of pattern recognition capabilities, ranging from diagnostics at the physician level to medical scene perception [2].
In contrast to traditional computer-aided diagnostic tools that rely heavily on supervised learning, recent advancements in self-supervised learning (SSL) have paved the way for reducing the dependence on large and annotated datasets [3, 4]. This innovative approach leverages unlabelled data to pretrain models, thus mitigating the need for extensive labelled data. In the field of medical image analysis, SSL has emerged as a promising technique, particularly for identifying complex features and lesions in medical images that require specialized expertise [5]. One prominent SSL approach that has gained significant traction in medical image analysis research is contrastive learning [6]. In 2020, Chen et al. introduced a simple framework for contrastive learning of visual representations (SimCLR) [14].
A base encoder is used to extract representation vectors from augmented data examples. The framework allows various selections of the network architecture without any constraints.
Projection head maps representations to the space where contrastive loss is applied.
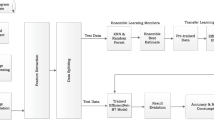
In our study, unlabelled NBI endoscopic images of colorectal neoplasia from PolypsSet [15] were used to train the SSL model. VGG16, MoblieNet, Resnet50, and Xception (initially trained on ImageNet) were loaded as the backbones of base encoders. We manually removed heads and added three fully connected layers (1*2048, 1*256, and 1*128) to the above backbones. In the SSL procedure, the last three convolutional layers and the three added fully connected layers were trainable, while the other layers of the backbones were nontrainable.
Fine-Tuning (Fig. 2)
After the initial training using the SSL model, the semi-supervised model was fine-tuned with the labelled NBI images of colorectal neoplasia from the PICCOLO dataset. Two extra fully connected layers (1*64 and 1*16) and a classifier were added. In the fine-tuning procedure, only the two newly added layers and the classifier were trainable for the target task of classification, while the others were nontrainable.
Supervised Transfer Learning (Fig. 3)
Supervised transfer learning was performed on the dataset as fine-tuning, i.e., the PICCOLO dataset. Like in the case of SSL, four other networks pretrained on ImageNet were loaded. Similarly, five fully connected layers (1*2048, 1*256, 1*128, 1*64, and 1*16) and a classifier were added to the backbones without a head. In the supervised transfer learning procedure, the last three convolutional layers, the five added fully connected layers, and the classifier were trainable, while the others were nontrainable.
Model Training
The Keras Python (version 3.8.0) platform (backbone: TensorFlow version 2.8.0) was used to train the models. Each image was resized to 224 × 224 pixels and input into the models in the form of RGB channels. The training parameters are listed in Supplementary Table 2. The training code for SSL was inspired by that of Sayak Paul, which is available at https://github.com/sayakpaul/SimCLR-in-TensorFlow-2. Our training code is available at https://osf.io/t3g8n.
Datasets
PolypsSet
Li et al. [15] collected various publicly available endoscopic datasets and a new dataset from the University of Kansas Medical Center to develop a relatively large endoscopic dataset for polyp detection and classification (https://doi.org/https://doi.org/10.7910/DVN/FCBUOR). The publicly available dataset includes 155 colorectal video sequences with 37,888 frames from the MICCAI 2017, CVC colon DB, and GLRC datasets [16]. NBI images were collected from the dataset to train the SSL model. To prevent duplication and ensure image quality, three endoscopists with more than 10 years of experience from Soochow University reviewed and finally selected 2000 unlabelled NBI images.
PICCOLO
This dataset contains 3433 images from clinical colonoscopy videos, including 2131 white light images and 1302 NBI images, from colonoscopy procedures in 40 human patients (https://www.biobancovasco.bioef.eus/en/Sample-and-data-catalog/Databases/PD178-PICCOLO-EN.html, Basque Biobank: https://labur.eus/EzJUN) [17]. To prevent duplication and ensure image quality, three endoscopists above reviewed and labelled 551 eligible NBI images based on the NICE classification (NICE I, n = 219; NICE II, n = 221; NICE III, n = 111). The labelled 551 endoscopic images were used to fine-tune the semi-supervised model. The detailed information on the two public datasets is presented in Supplementary Table 3.
Soochow University/Shanghai Jiao Tong University Dataset
A total of 1432 NBI images of colorectal neoplasia were collected from the First Affiliated Hospital of Soochow University and Kowloon Hospital of Shanghai Jiao Tong University. Three senior endoscopists independently reviewed and labelled 358 eligible images based on the NICE classification (NICE I, n = 126; NICE II, n = 109; NICE III, n = 123). The method for endoscopist reviewing and labelling is shown in Supplementary Fig. 1. The characteristics of colorectal neoplasia are listed in Supplementary Table 4. The dataset was used as an external test dataset. This study was approved by the ethics committee of the First Affiliated Hospital of Soochow University (approval number 2022098).
Human Endoscopists
To further evaluate the performances of the models, images from the test dataset (Soochow University/Shanghai Jiao Tong University) were evaluated by two independent endoscopists (junior, 3 years of endoscopic experience, and senior, more than 10 years of experience). They had not participated in reviewing or labelling the training images beforehand and were blind to the test set images. They were given 551 labelled images as a reference, then classified the 358 testing images independently. Moreover, to simulate a real clinical environment, two endoscopists were asked to complete the classification assignment within 1 h. A custom web interface was constructed to allow the reviewers to window, zoom, manipulate, and categorize each image.
Statistical Analysis
A confusion matrix was constructed and used to evaluate the performances of the models and endoscopists. TN, FN, TP, and FP indicate true negatives, false negatives, true positives, and false positives, respectively.
The accuracy represents the proportion of samples that were classified correctly among all samples.
The Matthew correlation coefficient (MCC) [18] measures the differences between the actual and predicted values. The MCC is the best single-value classification metric for summarizing the confusion matrix.
Cohen’s kappa [19] was used to measure the level of agreement between two raters or judges who each classified items into mutually exclusive categories.
A detailed explanation of MCC and Cohen’s Kappa is presented in the Supplementary Introduction.
Interpretation of Models
t-SNE Analysis
In this study, clustering patterns of predictions generated by models were visualized using t-SNE, an unsupervised technique for reducing the dimensionality of data [20]. By leveraging t-SNE with principal component analysis initialization, the high-dimensional vectors were processed and transformed into a two-dimensional visualization, revealing both the local structure and global geometry.
Grad-CAM
To enhance the interpretability of convolutional neural networks, Grad-CAM selectively highlights regions in input images that significantly contribute to prediction [21]. This technique could provide insights into how networks make decisions.
A detailed explanation of t-SNE and Grad-CAM is presented in the Supplementary Introduction.
Results
Performance of the Models
The four proposed semi-supervised learning (SimCLR) models with various backbones (VGG16, MobileNet, ResNet, and Xception), as well as models based on supervised transfer learning, were developed on the ternary task of the NICE classification. The reason for choosing the above four backbones is presented in the Supplementary Introduction. The performances of the eight models on the external test set are shown in Table 1.
Among the models, SimCLR-ResNet achieved the highest accuracy (0.908), followed by SimCLR-Xception (0.885), and SimCLR-MobileNet (0.872). The MCC and Cohen’s kappa of SimCLR-ResNet were 0.862 and 0.896 [0.857–0.945], respectively, which were greater than those of the other models. The confusion matrices are plotted in Fig. 4.
Comparison with Endoscopists
The performances of the junior and senior endoscopists are listed in Table 1, and their confusion matrices are provided in Fig. 4. The senior radiologist had a higher accuracy, MCC, and Cohen’s kappa coefficient (0.916, 0.875, and 0.944 [0.927–0.965], respectively, than did the SimCLR-ResNet. The junior radiologist had an accuracy, MCC, and Cohen’s kappa of 0.816, 0.724, and 0.863 [0.828–0.893], respectively, which are lower than those of the four SimCLR models.
Visualized Interpretation of the Models
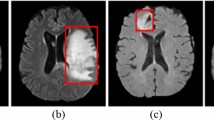
The outputs of the feature-extracted layers of supervised transfer learning and SimCLR were visualized by t-SNE, as shown in Fig. 5. The ternary samples showed better clustering through SSL in SimCLR than in supervised transfer learning. Furthermore, based on the outputs of SimCLR-ResNet, Grad-CAM was plotted, and an inferential explanation concerning the AI-inferred lesions is provided in Fig. 6 (the correct examples) and Fig. 7 (the erroneous examples).
Visualization of SimCLR-ResNet inference via Grad-CAM (the correct examples). The left column: the original endoscopic images. The middle column: heatmaps based on the output of the feature extractor’s last layer of SimCLR-ResNet. The right column: the Grad-CAM heatmap covering the original images, highlighting inferential explanations of the model
Visualization of SimCLR-ResNet inference via Grad-CAM (the incorrect examples). The left column: the original endoscopic images. The middle column: heatmaps based on the output of the feature extractor’s last layer of SimCLR-ResNet. The right column: the Grad-CAM heatmap covering the original images, in which the model mislocated the lesions
Discussion
In this study, we developed a series of SimCLR-based semi-supervised learning models to classify colorectal neoplasia based on the NICE classification. The ResNet-backboned SimCLR model showed an advantage over supervised transfer learning-based models and junior endoscopists, while it performed only slightly worse than senior endoscopists. The novel framework consists of (1) SSL on large unlabelled NBI-colonoscopic images and (2) fine-tuning on a few labelled images of colorectal neoplasia based on the NICE classification. Our study showed that, compared with traditional supervised learning, semi-supervised learning (which consists of SSL and fine-tuning) empowers AI models to achieve enhanced performances without relying solely on vast amounts of labelled data.
In the field of medical image analysis, deep learning has achieved remarkable performance in various competitive areas, such as pathology, radiology, and endoscopy [5, 22, 23]. However, the application of deep learning in low-resource settings faces challenges due to the scarcity of reliable labelled data [24]. SSL is now a novel solution for the successful use of various effective deep learning models [6]. These models are first pretrained without supervision using a source dataset and then fine-tuned for the target task [25]. Domain-specific SSL has proven to be effective at improving the medical image classification performance as compared to generic pretrained models [26]. Sun et al. used SimCLR as the backbone for a DL network to detect rib fractures from chest radiographs, showing superior detection sensitivity to traditional deep learning [8]. Ouyang et al. proposed a SimCLR-based self-supervised learning model to detect referable diabetic retinopathy, which overcame the training data insufficiency problem [27]. As demonstrated by the above studies, compared with traditional supervised learning, SSL can be used for computer-aided diagnosis of diseases that are difficult and time-consuming to label.
The application of SSL has shown notable promise in the detection, classification, and segmentation of medical images.
In the field of deep learning in gastrointestinal endoscopy, the current mainstream algorithm is still supervised learning [28,29,30]. Zhang et al. proposed a supervised-learning CNN model based on single-shot multibox detector architecture using 404 labelled endoscopic images with gastric polyps. The model realized real-time polyp detection with 50 frames per second and achieved 90.4% detection precision [31]. In recent years, with the introduction and maturation of self-supervised learning, it has gradually been applied in the field of endoscopy as well. We conducted a simple search for literature in the relevant field on PubMed, presented in Supplementary Table 5. In 2021, Du et al. [32] presented an SSL framework that employs an innovative module designed to generate efficient pairs for contrastive learning. By leveraging the similarity between images of the same lesion, this module enhanced the effectiveness of the contrastive learning process. Subsequently, an unsupervised approach was utilized to learn a visual feature representation that encapsulated the general features of esophageal endoscopic images. This representation was subsequently transferred to facilitate downstream esophageal disease classification tasks. Its results indicated that this framework achieved a classification accuracy surpassing that of other state-of-the-art semi-supervised methods. In 2023, in another study focused on Helicobacter pylori infection classification via blue laser endoscopic imaging, Jian et al. [33] proposed a self-supervised learning scheme consisting of an encoder and a prediction head. The encoder incorporated a backbone network, visual attention module, and feature fusion module to facilitate feature extraction through self-supervised contrastive learning. Once the encoder had been trained, the entire network was further fine-tuned using a small labelled image dataset. Through fivefold cross-validation experiments, it was observed that the proposed scheme achieved average F1-scores ranging from 0.885 to 0.915 for diagnosing H. pylori infection, outperforming existing methods. These diverse studies demonstrate that SSL techniques have shown potential in endoscopic diagnosis [6, 26]. The application of contrastive learning and self-supervised approaches has yielded notable improvements in accuracy and performance, addressing the challenge of limited labelled data.
In terms of the NICE classification, in 2022, Okamoto et al. [34] developed a supervised learning-based deep learning model for diagnosing colorectal lesions using the NICE classification. Coincidentally, ResNet was used as the backbone. Using a total of 4156 NBI images, the supervised learning model achieved a mean accuracy of 94.2%. In 2023, to address the challenge of limited labelled data availability, Krenzer et al. [30] proposed a few-shot learning (FSL) approach by creating an embedding space specifically tailored for colorectal lesions. FSL was designed to address the scarcity of labelled images [35]; specifically, in the transfer learning branch of FSL, the emphasis is on embedding learning. This process first involved training an embedding model that generates latent representations, allowing for task-specific notions of similarity between inputs to be quantified easily. Then, by structuring the latent space in such a way that samples from each class form distinct clusters, similarity metrics such as Euclidean or cosine distance can be used to determine the sample similarity and class affiliations. With this structural property, even with limited data available, simple class discrimination hypotheses can be constructed, such as k-nearest neighbor classification. In the study by Krenzer et al., the FSL-based model for NICE classification achieved an accuracy of 81.13%.
In this study, a SimCLR-based semi-supervised learning framework was developed to classify colorectal neoplasia using NBI colonoscopic images based on the NICE classification. Among the developed models, the ResNet-backboned SimCLR model exhibited better performance than the supervised transfer learning-based models. Given that the NICE classification was obtained through the consensus of three endoscopists, the classification performance was evaluated by junior and senior endoscopists independently. The semi-supervised learning model outperformed the endoscopist; however, it underperformed the senior endoscopist by 0.08% accuracy. In comparison with other computer-aided diagnosis methods for the NICE classification, our methods outperformed the FSL methods reported by Krenzer et al. [30] with almost 10% accuracy in datasets with scarce data. Moreover, the results of the proposed methods were similar to the results reported by Okamoto et al. [34], in which a total of 4156 NBI images were labelled for supervised learning. Finally, we visualized the advantage of SSL via t-SNE, which confirmed the improvement of the proposed framework.
There are several limitations in our study. First, despite quality review and selection of eligible images used for semi-supervised learning, the imperfections of the images are inevitable. It is possible that several NBI endoscopic images of a patient, captured from different angles, might have been included. Second, due to retrospective bias and the absence of clinical details and patients’ information in the public datasets, we failed to compare with datasets and provide more details. Third, the NICE classification system is determined by endoscopists under NBI endoscopy based on the color, microvascular structure, and surface pattern of the polyp. Its reliability is inferior to histopathology. Fourth, although we compared the classification performance of the supervised models and human endoscopists, the observational study of semi-supervised model-assisted compared with endoscopist-independent classification was not performed. Finally, further research and technology are required for real-time detection and classification to evaluate the performance of the semi-supervised model in clinical settings.
In this study, we presented a semi-supervised learning framework (SimCLR) for classifying colorectal neoplasia based on the NICE classification. Compared with traditional supervised learning, SSL empowers deep learning models to achieve improved performances with limited amounts of labelled endoscopic images.
Data Availability
The code used to train SimCLR models can be found on an open-accessed website (https://osf.io/t3g8n).
References
Fang S, et al.: Diagnosing and grading gastric atrophy and intestinal metaplasia using semi-supervised deep learning on pathological images: development and validation study. Gastric Cancer, 2023
Ouyang D, et al.: Electrocardiographic deep learning for predicting post-procedural mortality: a model development and validation study. Lancet Digit Health, 2023
Park S, Lee ES, Shin KS, Lee JE, Ye JC: Self-supervised multi-modal training from uncurated images and reports enables monitoring AI in radiology. Med Image Anal 91:103021, 2024
Azizi S, et al.: Robust and data-efficient generalization of self-supervised machine learning for diagnostic imaging. Nat Biomed Eng 7:756-779, 2023
Wang H, et al.: Scientific discovery in the age of artificial intelligence. Nature 620:47-60, 2023
Huang SC, Pareek A, Jensen M, Lungren MP, Yeung S, Chaudhari AS: Self-supervised learning for medical image classification: a systematic review and implementation guidelines. NPJ Digit Med 6:74, 2023
Chen T, Kornblith S, Norouzi M, Hinton GE: A Simple Framework for Contrastive Learning of Visual Representations. Ar**v abs/2002.05709, 2020
Sun H, et al.: Automated Rib Fracture Detection on Chest X-Ray Using Contrastive Learning. J Digit Imaging 36:2138-2147, 2023
Guha Roy A, et al.: Does your dermatology classifier know what it doesn’t know? Detecting the long-tail of unseen conditions. Med Image Anal 75:102274, 2022
Zhou Y, et al.: A foundation model for generalizable disease detection from retinal images. Nature 622:156-163, 2023
D**bachian R, Dube AJ, von Renteln D: Optical Diagnosis of Colorectal Polyps: Recent Developments. Curr Treat Options Gastroenterol 17:99-114, 2019
Houwen B, et al.: Definition of competence standards for optical diagnosis of diminutive colorectal polyps: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 54:88-99, 2022
Cocomazzi F, et al.: Accuracy and inter-observer agreement of the nice and kudo classifications of superficial colonic lesions: a comparative study. Int J Colorectal Dis 36:1561-1568, 2021
Chen T, Kornblith S, Norouzi M, Hinton G: A Simple Framework for Contrastive Learning of Visual Representations, 2020
Li K, et al.: Colonoscopy polyp detection and classification: Dataset creation and comparative evaluations. PLoS One 16:e0255809, 2021
Zhu S, et al.: Public Imaging Datasets of Gastrointestinal Endoscopy for Artificial Intelligence: a Review. J Digit Imaging 36:2578-2601, 2023
Sanchez-Peralta LF, et al.: PICCOLO White-Light and Narrow-Band Imaging Colonoscopic Dataset: A Performance Comparative of Models and Datasets. Applied Sciences 10:8501, 2020
Chicco D, Jurman G: The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 21:6, 2020
Um Y: Assessing Classification Accuracy using Cohen’s kappa in Data Mining. Journal of the Korea Society of Computer and Information 18, 2013
Liu J, Vinck M: Improved visualization of high-dimensional data using the distance-of-distance transformation. PLoS Comput Biol 18:e1010764, 2022
Wang Y, et al.: Automated Multimodal Machine Learning for Esophageal Variceal Bleeding Prediction Based on Endoscopy and Structured Data. J Digit Imaging 36:326-338, 2023
Yin M, et al.: Deep learning for pancreatic diseases based on endoscopic ultrasound: A systematic review. Int J Med Inform 174:105044, 2023
Jiang X, et al.: End-to-end prognostication in colorectal cancer by deep learning: a retrospective, multicentre study. Lancet Digit Health 6:e33-e43, 2024
Theodoris CV, et al.: Transfer learning enables predictions in network biology. Nature 618:616-624, 2023
Mukashyaka P, Sheridan TB, Foroughi Pour A, Chuang JH: SAMPLER: unsupervised representations for rapid analysis of whole slide tissue images. EBioMedicine 99:104908, 2023
Chen Y, Mancini M, Zhu X, Akata Z: Semi-Supervised and Unsupervised Deep Visual Learning: A Survey. IEEE Trans Pattern Anal Mach Intell PP, 2022
Ouyang J, Mao D, Guo Z, Liu S, Xu D, Wang W: Contrastive self-supervised learning for diabetic retinopathy early detection. Med Biol Eng Comput 61:2441-2452, 2023
Brand M, et al.: Development and evaluation of a deep learning model to improve the usability of polyp detection systems during interventions. United European Gastroenterol J 10:477-484, 2022
Krenzer A, et al.: A Real-Time Polyp-Detection System with Clinical Application in Colonoscopy Using Deep Convolutional Neural Networks. J Imaging 9, 2023
Krenzer A, et al.: Automated classification of polyps using deep learning architectures and few-shot learning. BMC Med Imaging 23:59, 2023
Zhang X, et al.: Real-time gastric polyp detection using convolutional neural networks. PLoS One 14:e0214133, 2019
Du W, et al.: Improving the Classification Performance of Esophageal Disease on Small Dataset by Semi-supervised Efficient Contrastive Learning. J Med Syst 46:4, 2021
Jian G-Z, Lin G-S, Wang C-M, Yan S-L: Classification of Helicobacter Pylori infection based on deep convolutional neural network with visual attention and self-supervised learning for endoscopic images
Okamoto Y, et al.: Development of multi-class computer-aided diagnostic systems using the NICE/JNET classifications for colorectal lesions. J Gastroenterol Hepatol 37:104-110, 2022
Yin M, et al.: Identification of gastric signet ring cell carcinoma based on endoscopic images using few-shot learning. Dig Liver Dis, 2023
Acknowledgements
The authors thank all the participating patients and investigators.
Funding
This study was supported by the National Natural Science Foundation of China (82000540), Suzhou Clinical Center of Digestive Diseases (Szlcyxzx202101), Youth Program of Suzhou Health Committee (KJXW2019001), Open Fund of Key Laboratory of Hepatosplenic Surgery, Ministry of Education, Harbin, China (GPKF202304), Frontier Technologies of Science and Technology Projects of Changzhou Municipal Health Commission (QY202309), Medical Education Collaborative Innovation Fund of Jiangsu University (no. JDY2022018), and Changzhou Municipal Health Commission Science and Technology Project (QN202139).
Author information
Authors and Affiliations
Contributions
Wang Y., Ni H., Liu L., and Zhu S. contributed to manuscript drafting and data analysis. Zhou J., Lin J., Yin M., and Gao J. contributed to data acquisition. Zhou J. and Yin Q. contributed to the manuscript revision. Li R. and Liu L. contributed to endoscopic image reading. Li R. and Zhu J. contributed to the design of the work. All authors contributed to the final approval of the completed version.
Corresponding authors
Ethics declarations
Ethics Approval
This retrospective study was approved by the ethics committee of the First Affiliated Hospital of Soochow University (approval number 2022098).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Ni, H., Zhou, J. et al. A Semi-Supervised Learning Framework for Classifying Colorectal Neoplasia Based on the NICE Classification. J Digit Imaging. Inform. med. (2024). https://doi.org/10.1007/s10278-024-01123-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10278-024-01123-9