Abstract
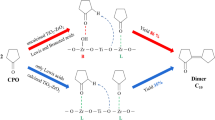
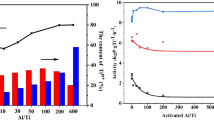
A series of TiO2/Al2O3 catalysts for cyclohexanone condensation have been prepared by impregnation method. Various characterization techniques, including XRD, BET, SEM, FT-IR, NH3-TPD, and Py-IR were applied to characterize their physical properties and chemical structures. The results show that the acidity of the TiO2/Al2O3 catalysts are all come from L-acid. In addition, the effects of reaction parameters such as TiO2 loading dosage, amount of cyclohexane, catalyst dosage, particle mesh, agitation rate, temperature and time were examined. The optimal reaction conditions were 40 ~ 60 particle mesh, 8% catalyst dosage, 393.15 K reaction temperature, 300 r/min agitation rate for 120 min with the conversion of cyclohexanone is 29.11% and close to 100% ultrahigh selectivity. Five cycles of reaction suggested the activity and selectivity of the catalyst did not change obviously and had potential industrial application value. Finally, the reaction kinetics was evaluated in cyclohexane under the reaction conditions of particle mesh 40 ~ 60 and 8% catalyst dosage and absence removing water produced, indicating the cyclohexanone self-condensation reaction was a second-order reaction.






Similar content being viewed by others
References
Bhuyan D, Kalita SJ, Saikia L (2022) Mesoporous SBA-15 supported gold nanoparticles for solvent-free oxidation of cyclohexane: superior catalytic activity with higher cyclohexanone selectivity. Phys Chem Chem Phys 24:29781–29790. https://doi.org/10.1039/d2cp04198g
Yin D, Ji R, Lv F et al (2023) Highly efficient conversion of phenol to cyclohexanone on Pd-based catalysts by cobalt do**. Fuel. https://doi.org/10.1016/j.fuel.2022.126060
Peng X, Zeb S, Zhao J et al (2020) Highly selective self-condensation of cyclohexanone: the distinct catalytic behaviour of HRF5015. R Soc Open Sci. https://doi.org/10.1098/rsos.200123
Bai P, Wu P, Yan Z et al (2009) Cation–anion double hydrolysis derived mesoporous γ-Al2O3 as an environmentally friendly and efficient aldol reaction catalyst. J Mater Chem. https://doi.org/10.1039/b819993k
Zeng Y, Zhang T, Xu Y et al (2016) Cu/Mg/Al/Zr non-noble metal catalysts for o-phenylphenol synthesis. RSC Adv 6:6737–6746. https://doi.org/10.1039/c5ra25340c
Zeng Y, Zhang T, Xu Y et al (2016) Cu/Mg/Al hydrotalcite-like hydroxide catalysts for o-phenylphenol synthesis. Appl Clay Sci 126:207–214. https://doi.org/10.1016/j.clay.2016.03.017
Wang J, Zhang T, Li K et al (2018) Dehydrogenation catalysts for synthesis of o-phenylphenol via Cu/Ni/Mg/Al hydrotalcite-like compounds as precursors. Catalysts. https://doi.org/10.3390/catal8050186
Zhang H, Gong X, Li Z et al (2021) Synthesis of new phosphorous-containing flame retardant and the properties of flame retardant epoxy resins. Pigm Resin Technol 50:554–562. https://doi.org/10.1108/prt-09-2020-0101
Chen Y, Yuan S, Yin H et al (2011) Kinetics of the reversible dimerization reaction of cyclohexanone over gamma-alumina catalyst. React Kinet Mech Catal 102:183–194. https://doi.org/10.1007/s11144-010-0250-7
Lorenzo D, Santos A, Simon E et al (2013) Kinetic of alkali catalyzed self-condensation of cyclohexanone. Ind Eng Chem Res 52:2257–2265. https://doi.org/10.1021/ie303213p
Mahajan YS, Kamath RS, Kumbhar PS et al (2008) Self-condensation of cyclohexanone over ion exchange resin catalysts: kinetics and selectivity aspects. Ind Eng Chem Res 47:25–33. https://doi.org/10.1021/ie061275b
Aragon JM, Vegas JMR, Jodra LG (1993) Catalytic behavior of macroporous resins in catalytic processes with water production-activation and inhibition effects in the kinetics of the self-condensation of cyclohexanone. Ind Eng Chem Res 32:2555–2562. https://doi.org/10.1021/ie00023a019
Chen Y, Yuan S, Yin H et al (2010) Kinetics of the reversible dimerization reaction of cyclohexanone over γ-alumina catalyst. React Kinet Mech Catal 102:183–194. https://doi.org/10.1007/s11144-010-0250-7
Sharma LK, Kim KB, Elliott GI (2011) A selective solvent-free self-condensation of carbonyl compounds utilizing microwave irradiation. Green Chem 13:1546–1549. https://doi.org/10.1039/c1gc15164a
Liang J, Li X, Liu C et al (2022) SCM-39, the direct synthesized ATS aluminosilicate zeolite as a promising solid acid catalyst. Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2021.111608
Deng Q, Nie G, Pan L et al (2015) Highly selective self-condensation of cyclic ketones using MOF-encapsulating phosphotungstic acid for renewable high-density fuel. Green Chem 17:4473–4481. https://doi.org/10.1039/c5gc01287b
Ai M (2005) Formation of methyl methacrylate by condensation of methyl propionate with formaldehyde over silica-supported cesium hydroxide catalysts. Appl Catal a-Gen 288:211–215. https://doi.org/10.1016/j.apcata.2005.04.027
Bai P, Wu P, Zhao G et al (2008) Cation–anion double hydrolysis derived mesoporous γ-Al2O3as an environmentally friendly and efficient aldol reaction catalyst. J Mater Chem 18:74–76. https://doi.org/10.1039/b713283b
Santes V, Herbert J, Cortez MT et al (2005) Catalytic hydrotreating of heavy gasoil FCC feed on alumina-titania- supported NiMo catalysts. Appl Catal a-Gen 281:121–128. https://doi.org/10.1016/j.apcata.2004.11.025
Vazquez-Garrido I, Lopez-Benitez A, Berhault G et al (2019) Effect of support on the acidity of NiMo/Al2O3-MgO and NiMo/TiO2-Al2O3 catalysts and on the resulting competitive hydrodesulfurization/hydrodenitrogenation reactions. Fuel 236:55–64. https://doi.org/10.1016/j.fuel.2018.08.053
Jiang F, Zeng L, Li SR et al (2015) Propane dehydrogenation over Pt/TiO2-Al2O3 catalysts. ACS Catal 5:438–447. https://doi.org/10.1021/cs501279v
Lorenzo D, Simón E, Santos A et al (2014) Kinetic model of catalytic self-condensation of cyclohexanone over amberlyst 15. Ind Eng Chem Res 53:19117–19127. https://doi.org/10.1021/ie5032265
Pan K, Wang F, Wei S et al (2020) Low-temperature solution synthesis and characterization of enhanced titanium dioxide photocatalyst on tailored mesoporous gamma-Al2O3 support. Compos Commun 19:82–89. https://doi.org/10.1016/j.coco.2020.02.009
Song H, Dai M, Guo Y-T et al (2012) Preparation of composite TiO2-Al2O3 supported nickel phosphide hydrotreating catalysts and catalytic activity for hydrodesulfurization of dibenzothiophene. Fuel Process Technol 96:228–236. https://doi.org/10.1016/j.fuproc.2012.01.001
Sedneva TA, Lokshin EP, Belikov ML et al (2013) Preparation and characterization of mesoporous TiO2-Al2O3 composites. Inorg Mater 49:786–794. https://doi.org/10.1134/s0020168513080141
Nasirmahale LN, Shirini F, Bayat Y et al (2023) Solvent-free synthesis of imidazo 1,2-a pyrimidine-3-carbonitriles and 1,2,4-triazolo 4,3-a pyrimidines under the catalytic performance of TiO2-bip-NH2+ C(NO2)3- as a novel nanocatalyst. J Mol Struct. https://doi.org/10.1016/j.molstruc.2022.134210
Huang W, Zhou Y, Wei Q et al (2022) Synthesis of mesoporous TiO2-Al2O3 composites supported NiW hydrotreating catalysts and their superior catalytic performance for heavy oil hydrodenitrogenation. Fuel. https://doi.org/10.1016/j.fuel.2022.123802
Zarei S, Farhadian N, Akbarzadeh R et al (2020) Fabrication of novel 2D Ag-TiO2/gamma-Al2O3/Chitosan nano-composite photocatalyst toward enhanced photocatalytic reduction of nitrate. Int J Biol Macromol 145:926–935. https://doi.org/10.1016/j.ijbiomac.2019.09.183
Khom-in J, Praserthdam P, Panpranot J et al (2008) Dehydration of methanol to dimethyl ether over nanocrystalline Al2O3 with mixed γ- and χ-crystalline phases. Catal Commun 9:1955–1958. https://doi.org/10.1016/j.catcom.2008.03.009
Otroshchenko TP, Rodemerck U, Linke D et al (2017) Synergy effect between Zr and Cr active sites in binary CrZrOx or supported CrOx/LaZrOx: Consequences for catalyst activity, selectivity and durability in non-oxidative propane dehydrogenation. J Catal 356:197–205. https://doi.org/10.1016/j.jcat.2017.10.012
Zhu Q, Duan H, Lin B et al (2019) Higher acetone conversion obtained over a TiO2-Pd bifunctional catalyst for liquid-phase synthesis of methyl isobutyl ketone: the role of Al2O3 support. Catal Lett 149:2636–2644. https://doi.org/10.1007/s10562-019-02861-0
Yadav R, Muralidhar A, Shamna A et al (2018) Aluminium oxide supported on SBA-15 molecular sieves as potential lewis acid catalysts for epoxide ring opening using aniline. Catal Lett 148:1407–1415. https://doi.org/10.1007/s10562-018-2366-8
Bao Q, Bu T, Yan J et al (2017) Synthesis of methyl acrylate by aldol condensation of methyl acetate with formaldehyde Over Al2O3-supported barium catalyst. Catal Lett 147:1540–1550. https://doi.org/10.1007/s10562-017-2014-8
Funding
Natural Science Research of Jiangsu Higher Education Institutions of China, 20110101, **hua Liang.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Y., Ren, X., Liu, B. et al. Ultrahigh selectivity self-condensation of cyclohexanone over TiO2/Al2O3 catalysts and kinetics study. Reac Kinet Mech Cat 136, 2071–2087 (2023). https://doi.org/10.1007/s11144-023-02434-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02434-8