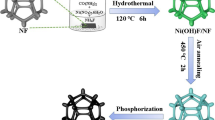
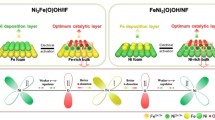
Abstract
Nitride modification is focused on boosting reaction kinetics by optimizing the hydrogen evolution reaction (HER) pathway of nitrides. This study adopted a creative co-do** strategy of using a P-anion and a Ce-cation to construct a P,Ce-FeNi3N/nickel foam (NF) electrode. At an overpotential of 200 mV, the well-designed P,Ce-FeNi3N/NF electrode showed a current density of 340 mA cm−2, which was twice that of the commercial Pt/C@NF electrode (174 mA cm−2). Theoretical calculations revealed that unlike the single Ni active site in FeNi3N/NF, P,Ce-FeNi3N/NF possessed two active sites Ni and P. As a result, reaction kinetics during the alkaline HER process was considerably optimized. Furthermore, the assembled NiFeCe-layered double hydroxides/NF‖P,Ce-FeNi3N/NF cell only needed a voltage of 1.537 V to achieve a high current density of 500 mA cm−2. Overall, this study provides a new strategy for achieving superior electrocatalytic performance of nitrides by optimizing and improving reaction kinetics.
摘要
通过优化氮化物的析氢反应(HER)途径来提高反应动力学是氮 化物改性的重点. 本工作创造性地采用P-阴离子和Ce-阳离子的共掺杂 策略构建了P,Ce-FeNi3 N/NF电极. 该P,Ce-FeNi3 N/NF电极在200 mV过 电位下的电流密度(340 mA cm −2) 是商业Pt/C@NF 电流密度 (174 mA cm−2)的两倍. 理论计算表明, 与FeNi3 N/NF的单个Ni活性位点 不同, P,Ce-FeNi3 N/NF 利用双活性位点(Ni和P)机制极大地优化了碱性 HER过程中的反应动力学. 此外, 组装的NiFeCe-LDH/NF‖ P,Ce-FeNi3 N/NF电池仅需要1.537 V的电压即可实现500 mA cm−2 的高电流 密度. 这项工作从反应路径优化和反应动力学改进的角度为实现氮化 物优异的电催化性能提供了一种新策略.

Similar content being viewed by others
References
Gu Y, Wu A, Jiao Y, et al. Two-dimensional porous molybdenum phosphide/nitride heterojunction nanosheets for pH-universal hydrogen evolution reaction. Angew Chem Int Ed, 2021, 60: 6673–6681
Ye TN, Park SW, Lu Y, et al. Contribution of nitrogen vacancies to ammonia synthesis over metal nitride catalysts. J Am Chem Soc, 2020, 142: 14374–14383
Gao X, Feng J, Su D, et al. In-situ exfoliation of porous carbon nitride nanosheets for enhanced hydrogen evolution. Nano Energy, 2019, 59: 598–609
Yu L, Zhu Q, Song S, et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat Commun, 2019, 10: 5106
**g L, Tian Q, Li X, et al. Dual-engineering of porous structure and carbon edge enables highly selective H2O2 electrosynthesis. Adv Funct Mater, 2023, 2305795
Kao CC, Ye C, Hao J, et al. Suppressing hydrogen evolution via anticatalytic interfaces toward highly efficient aqueous Zn-ion batteries. ACS Nano, 2023, 17: 3948–3957
Guo J, Zheng Y, Hu Z, et al. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat Energy, 2023, 8: 264–272
Yin K, Chao Y, Lv F, et al. One nanometer PtIr nanowires as high-efficiency bifunctional catalysts for electrosynthesis ofethanol into high value-added multicarbon compound coupled with hydrogen production. J Am Chem Soc, 2021, 143: 10822–10827
Zeng L, Zhao Z, Lv F, et al. Anti-dissolution Pt single site with Pt(OH)(O3)/Co(P) coordination for efficient alkaline water splitting electrolyzer. Nat Commun, 2022, 13: 3822
Liu X, Jiao Y, Zheng Y, et al. Mechanism of C–N bonds formation in electrocatalytic urea production revealed by ab initio molecular dynamics simulation. Nat Commun, 2022, 13: 5471
Wei X, Liu Y, Zhu X, et al. Dynamic reconstitution between copper single atoms and clusters for electrocatalytic urea synthesis. Adv Mater, 2023, 35: 2300020
Wang T, Huang Z, Liu T, et al. Transforming electrocatalytic biomass upgrading and hydrogen production from electricity input to electricity output. Angew Chem Int Ed, 2022, 61: e202115636
Wang T, Tao L, Zhu X, et al. Combined anodic and cathodic hydrogen production from aldehyde oxidation and hydrogen evolution reaction. Nat Catal, 2022, 5: 66–73
Luo H, Wang K, Lin F, et al. Amorphous MoOx with high oxophilicity interfaced with PtMo alloy nanoparticles boosts anti-CO hydrogen electrocatalysis. Adv Mater, 2023, 35: 2211854
Jeong HY, Oh J, Yi GS, et al. High-performance water electrolyzer with minimum platinum group metal usage: Iron nitride-iridium oxide core-shell nanostructures for stable and efficient oxygen evolution reaction. Appl Catal B-Environ, 2023, 330: 122596
Hu Q, Han Z, Wang X, et al. Facile synthesis of sub-nanometric copper clusters by double confinement enables selective reduction of carbon dioxide to methane. Angew Chem Int Ed, 2020, 59: 19054–19059
Dan Z, Liang W, Gong X, et al. Substitutional do** engineering toward W2N nanorod for hydrogen evolution reaction at high current density. ACS Mater Lett, 2022, 4: 1374–1380
Wu Z, Zhao Y, **ao W, et al. Metallic-bonded Pt–Co for atomically dispersed Pt in the Co4N matrix as an efficient electrocatalyst for hydrogen generation. ACS Nano, 2022, 16: 18038–18047
Huang L, Yao R, Wang X, et al. In situ phosphating of Zn-doped bimetallic skeletons as a versatile electrocatalyst for water splitting. Energy Environ Sci, 2022, 15: 2425–2434
Li P, Chen R, Zhao S, et al. Architecture control and electronic structure engineering over Ni-based nitride nanocomposite for boosting ammonia borane dehydrogenation. Appl Catal B-Environ, 2021, 298: 120523
Doan TLL, Nguyen DC, Prabhakaran S, et al. Single-atom co-decorated MoS2 nanosheets assembled on metal nitride nanorod arrays as an efficient bifunctional electrocatalyst for pH-universal water splitting. Adv Funct Mater, 2021, 31: 2100233
Lin F, Dong Z, Yao Y, et al. Electrocatalytic hydrogen evolution of ultrathin Co-Mo5N6 heterojunction with interfacial electron redistribution. Adv Energy Mater, 2020, 10: 2002176
Lee SY, Oh HJ, Kim MJ, et al. Insights into enhanced activity and durability of hierarchical Fe-doped Ni(OH)2/Ni catalysts for alkaline oxygen evolution reaction: In situ XANES studies. Appl Catal B-Environ, 2023, 324: 122269
Sun Y, Wang J, Qi Y, et al. Efficient electrooxidation of 5-hydroxymethylfurfural using Co-doped Ni3S2 catalyst: Promising for H2 production under industrial-level current density. Adv Sci, 2022, 9: 2200957
Wang T, Wang P, Zang W, et al. Nanoframes of Co3O4-Mo2N heterointerfaces enable high-performance bifunctionality toward both electrocatalytic HER and OER. Adv Funct Mater, 2022, 32: 2107382
Yin Z, Sun Y, Jiang Y, et al. Hierarchical cobalt-doped molybdenum-nickel nitride nanowires as multifunctional electrocatalysts. ACS Appl Mater Interfaces, 2019, 11: 27751–27759
Chen Y, Yu J, Jia J, et al. Metallic Ni3Mo3N porous microrods with abundant catalytic sites as efficient electrocatalyst for large current density and superstability of hydrogen evolution reaction and water splitting. Appl Catal B-Environ, 2020, 272: 118956
Ma R, Cui X, Xu X, et al. Collaborative integration of ultrafine Fe2P nanocrystals into Fe, N, P-codoped carbon nanoshells for highly-efficient oxygen reduction. Nano Energy, 2023, 108: 108179
Yao N, Li P, Zhou Z, et al. Synergistically tuning water and hydrogen binding abilities over Co4N by Cr do** for exceptional alkaline hydrogen evolution electrocatalysis. Adv Energy Mater, 2019, 9: 1902449
Wang J, Sun Y, Qi Y, et al. Vanadium-do** and interface engineering for synergistically enhanced electrochemical overall water splitting and urea electrolysis. ACS Appl Mater Interfaces, 2021, 13: 57392–57402
Guo D, Zeng Z, Wan Z, et al. A CoN-based OER electrocatalyst capable in neutral medium: Atomic layer deposition as rational strategy for fabrication. Adv Funct Mater, 2021, 31: 2101324
Li F, Jiang M, Lai C, et al. Yttrium- and cerium-codoped ultrathin metal-organic framework nanosheet arrays for high-efficiency electrocatalytic overall water splitting. Nano Lett, 2022, 22: 7238–7245
Zhou X, Mo Y, Yu F, et al. Engineering active iron sites on nanoporous bimetal phosphide/nitride heterostructure array enabling robust overall water splitting. Adv Funct Mater, 2023, 33: 2209465
Zhu Z, Liu Z, Zhao R, et al. Heterogeneous nitride interface enabled by stepwise-reduction electrolyte design for dense Li deposition in carbonate electrolytes. Adv Funct Mater, 2022, 32: 2209384
Ban J, Xu H, Cao G, et al. Synergistic effects of phase transition and electron-spin regulation on the electrocatalysis performance of ternary nitride. Adv Funct Mater, 2023, 33: 2300623
Deng C, Toe CY, Li X, et al. Earth-abundant metal-based electrocatalysts promoted anodic reaction in hybrid water electrolysis for efficient hydrogen production: Recent progress and perspectives. Adv Energy Mater, 2022, 12: 2201047
Kou Z, Wang T, Gu Q, et al. Rational design of holey 2D nonlayered transition metal carbide/nitride heterostructure nanosheets for highly efficient water oxidation. Adv Energy Mater, 2019, 9: 1803768
Dai L, Yao F, Yu L, et al. Boosting alkaline hydrogen evolution on stoichiometric molybdenum carbonitride via an interstitial vacancy-elimination strategy. Adv Energy Mater, 2022, 12: 2200974
Li G, Yu J, Yu W, et al. Phosphorus-doped iron nitride nanoparticles encapsulated by nitrogen-doped carbon nanosheets on iron foam in situ derived from saccharomycetes cerevisiae for electrocatalytic overall water splitting. Small, 2020, 16: 2001980
Yang Y, Wang Y, Zhao L, et al. Visualizing nucleation and growth process of vanadium-supramolecular nanoribbons self-assembled by rapid cooling method towards high-capacity vanadium nitride anode materials. Adv Energy Mater, 2022, 12: 2103158
Zhang Y, Jiang S, Li Y, et al. In situ grown hierarchical electrospun nanofiber skeletons with embedded vanadium nitride nanograins for ultra-fast and super-long cycle life aqueous Zn-ion batteries. Adv Energy Mater, 2022, 13: 2202826
Guo K, Fan D, Bao J, et al. Atomic-level phosphorus-doped ultrathin Pt nanodendrites as efficient electrocatalysts. Adv Funct Mater, 2022, 32: 2208057
Song D, Sun J, Sun L, et al. Acidic media regulated hierarchical cobalt compounds with phosphorous do** as water splitting electro-catalysts. Adv Energy Mater, 2021, 11: 2100358
** H, Wang X, Tang C, et al. Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Adv Mater, 2021, 33: 2007508
Lin X, Cao S, Chen X, et al. Two birds with one stone: Contemporaneously boosting OER activity and kinetics for layered double hydroxide inspired by photosystem II. Adv Funct Mater, 2022, 32: 2202072
Zhang S, Yao Y, Jiao X, et al. Mo2N-W2N heterostructures embedded in spherical carbon superstructure as highly efficient polysulfide electrocatalysts for stable room-temperature Na–S batteries. Adv Mater, 2021, 33: 2103846
Li X, Zhang H, Hu Q, et al. Amorphous NiFe oxide-based nanoreactors for efficient electrocatalytic water oxidation. Angew Chem Int Ed, 2023, 62: e202300478
You H, Wu D, Si D, et al. Monolayer NiIr-layered double hydroxide as a long-lived efficient oxygen evolution catalyst for seawater splitting. J Am Chem Soc, 2022, 144: 9254–9263
Sun Z, Lin L, He J, et al. Regulating the spin state of Fe111 enhances the magnetic effect of the molecular catalysis mechanism. J Am Chem Soc, 2022, 144: 8204–8213
Wu T, Song E, Zhang S, et al. Engineering metallic heterostructure based on Ni3N and 2M-MoS2 for alkaline water electrolysis with industry-compatible current density and stability. Adv Mater, 2022, 34: 2108505
Yang Z, Chen C, Zhao Y, et al. Pt single atoms on CrN nanoparticles deliver outstanding activity and CO tolerance in the hydrogen oxidation reaction. Adv Mater, 2023, 35: 2208799
Li J, **a Z, Xue Q, et al. Insights into the interfacial lewis acid-base pairs in CeO2-loaded CoS2 electrocatalysts for alkaline hydrogen evolution. Small, 2021, 17: 2103018
Rosmini C, Tsoncheva T, Kovatcheva D, et al. Mesoporous Ce-Fe-Ni nanocomposites encapsulated in carbon-nanofibers: Synthesis, characterization and catalytic behavior in oxygen evolution reaction. Carbon, 2022, 196: 186–202
Zhou P, Hai G, Zhao G, et al. CeO2 as an “electron pump” to boost the performance of Co4N in electrocatalytic hydrogen evolution, oxygen evolution and biomass oxidation valorization. Appl Catal B-Environ, 2023, 325: 122364
Li RQ, Zeng S, Sang B, et al. Regulating electronic structure of porous nickel nitride nanosheet arrays by cerium do** for energy-saving hydrogen production coupling hydrazine oxidation. Nano Res, 2023, 16: 2543–2550
Hu Q, Gao K, Wang X, et al. Subnanometric Ru clusters with upshifted d band center improve performance for alkaline hydrogen evolution reaction. Nat Commun, 2022, 13: 3958
Zhang N, Yi Y, Lian J, et al. Effects of Ce do** on the Fenton-like reactivity of Cu-based catalyst to the fluconazole. Chem Eng J, 2020, 395: 124897
Gao W, Wu Y, Wan X, et al. Engineering the electronic structure of FeP with rare earth elements to enhance the electrocatalytic hydrogen evolution performance. J Mater Chem A, 2023, 11: 18126–18134
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52072197 and 21971132), the 111 Project of China (D20017), the Outstanding Youth Foundation of Shandong Province, China (ZR2019JQ14), the Natural Science Foundation of Shandong Province, China (ZR2022QE098), the Major Scientific and Technological Innovation Project (2019JZZY020405), the Major Basic Research Program of Natural Science Foundation of Shandong Province (ZR2020ZD09), the Postdoctoral Innovation Project of Shandong Province (SDCX-ZG-20220307), Qingdao Postdoctoral Researcher Applied Research Project (04030431060100), and “Double-Hundred Talent Plan” of Shandong Province (WST2020003).
Author information
Authors and Affiliations
Contributions
Author contributions Li S and Du Y designed the experiment. Li S prepared the samples and performed the characterizations. All authors contributed to the discussion of the results. Li S and Du Y participated in writing and revision of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper.
Shuangshuang Li is a graduate student at the School of Environment, Qingdao University of Science and Technology. Her research interest is the design of nitride-based catalysts and their electrocatalytic performance.
Yunmei Du is currently a postdoctoral fellow at the School of Environment and Safety, Qingdao University of Science and Technology. In recent years, he has been committed to the design and construction of heterogeneous catalysts and their applications in the field of electrocatalysis.
Lei Wang is a professor of the School of Environment, Qingdao University of Science and Technology. In 2006, he graduated from the State Key Laboratory of Inorganic Synthesis and Preparation Chemistry, Jilin University with a PhD degree. In recent years, his research has been focused on the synthesis methods of inorganic micro-nano materials and their applications in energy storage and conversion.
Supporting Information
40843_2023_2632_MOESM1_ESM.pdf
Optimizing the reaction pathway of nitride electrode by co-do** strategy for boosting alkaline hydrogen evolution reaction kinetics
Rights and permissions
About this article
Cite this article
Li, S., Du, Y., Wang, M. et al. Optimizing the reaction pathway of nitride electrode by co-do** strategy for boosting alkaline hydrogen evolution reaction kinetics. Sci. China Mater. 66, 4639–4649 (2023). https://doi.org/10.1007/s40843-023-2632-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2632-y