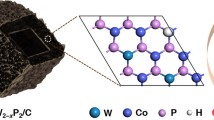
Abstract
Ruthenium phosphide is a promising catalyst for hydrogen evolution due to its cost-effectiveness compared to platinum. However it faces the challenge of having a high binding energy for hydrogen intermediates. In this study, we demonstrate that the incorporation of iridium in ruthenium phosphides lowers the binding energy of hydrogen intermediates, thereby controlling the overpotential and Tafel slope of hydrogen evolution. When the Ir content was doped at 3 at.%, the catalyst achieved an overpotential of 33 mV and a Tafel slope of 33 mV dec−1 under acidic conditions, which are similar to those of the benchmark Pt/C catalyst. In situ Raman spectroscopy and density functional theory (DFT) calculations suggest that the enhanced catalytic activity originates from the near-neutral Gibbs free energy of hydrogen adsorption on the hollow site of the iridium cluster implanted onto ruthenium phosphide.
Graphical Abstract







Similar content being viewed by others
Data availability
Data are available on request from the authors.
References
Esposito DV, Hunt ST, Kimmel YC, Chen JG. A new class of electrocatalysts for hydrogen production from water electrolysis: metal monolayers supported on low-cost transition metal carbides. J Am Chem Soc. 2012;134:3025.
Zeng M, Li Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J Mater Chem A. 2015;3:14942.
Yang Y, Yu Y, Li J, Chen Q, Du Y, Rao P, Li R, Jia C, Kang Z, Deng P. Engineering ruthenium-based electrocatalysts for effective hydrogen evolution reaction. Nano-Micro Lett. 2021;13:1.
Bae S-Y, Mahmood J, Jeon I-Y, Baek J-B. Recent advances in ruthenium-based electrocatalysts for the hydrogen evolution reaction. Nanoscale Horiz. 2020;5:43.
**ao P, Chen W, Wang X. A review of phosphide-based materials for electrocatalytic hydrogen evolution. Adv Energy Mater. 2015;5:1500985.
Kumar A, Kim I-H, Mathur L, Kim H-S, Song S-J. Design of tin polyphosphate for hydrogen evolution reaction and supercapacitor applications. J Korean Ceram Soc. 2021;58:688.
Wang Y, Liu Z, Liu H, Suen NT, Yu X, Feng L. Electrochemical hydrogen evolution reaction efficiently catalyzed by Ru2P nanoparticles. Chemsuschem. 2018;11:2724.
Liu T, Wang J, Zhong C, Lu S, Yang W, Liu J, Hu W, Li CM. Benchmarking three ruthenium phosphide phases for electrocatalysis of the hydrogen evolution reaction: experimental and theoretical insights. Chem Eur J. 2019;25:7826.
Cherevko S, Geiger S, Kasian O, Kulyk N, Grote J-P, Savan A, Shrestha BR, Merzlikin S, Breitbach B, Ludwig A. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: a comparative study on activity and stability. Catal Today. 2016;262:170.
Bhunia K, Chandra M, Sharma SK, Pradhan D, Kim S-J. A critical review on transition metal phosphide based catalyst for electrochemical hydrogen evolution reaction: Gibbs free energy, composition, stability, and true identity of active site. Coord Chem Rev. 2023;478:214956.
Jiang X, Jang H, Liu S, Li Z, Kim MG, Li C, Qin Q, Liu X, Cho J. The heterostructure of Ru2P/WO3/NPC synergistically promotes H2O dissociation for improved hydrogen evolution. Angew Chem Int Ed. 2021;60:4110.
Wang P, Zhang X, Zhang J, Wan S, Guo S, Lu G, Yao J, Huang X. Precise tuning in platinum-nickel/nickel sulfide interface nanowires for synergistic hydrogen evolution catalysis. Nat Commun. 2017;8:14580.
Roy SB, Moon S, Patil A, Rehman MA, Yoo S, Seo Y, Park JH, Kang K, Jun SC. Tuning the band (p and d) center and enhancing the active sites by nitrogen (N) do** on iridium diphosphide (IrP2) for accelerating pH-universal water electrolysis. Appl Catal B Environ. 2022;319:121906.
Chen D, Yu R, Lu R, Pu Z, Wang P, Zhu J, Ji P, Wu D, Wu J, Zhao Y, Kou Z, Yu J, Mu S. Tunable Ru-Ru2P heterostructures with charge redistribution for efficient pH-universal hydrogen evolution. InfoMater. 2022;4:e12287.
Zhu J, Li S, **ao M, Zhao X, Li G, Bai Z, Li M, Hu Y, Feng R, Liu W. Tensile-strained ruthenium phosphide by anion substitution for highly active and durable hydrogen evolution. Nano Energy. 2020;77:105212.
Yang D, Li P, Gao X-Y, Han J, Liu Z-Y, Yang Y-P, Yang J-H. Modulating surface segregation of Ni2P-Ru2P/CCG nanoparticles for boosting hydrogen evolution reaction in pH-universal. Chem Eng J. 2022;432:134422.
Deng Y, Liu Z, Wang A, Sun D, Chen Y, Yang L, Pang J, Li H, Li H, Liu H. Oxygen-incorporated MoX (X: S, Se or P) nanosheets via universal and controlled electrochemical anodic activation for enhanced hydrogen evolution activity. Nano Energy. 2019;62:338.
Chen Y, Wang D, Meng T, ** endows cobalt phosphide nanowires with enhanced alkaline hydrogen evolution activity. ACS Appl Energy Mater. 2022;5:697.
Zhang X-L, Yu P-C, Su X-Z, Hu S-J, Shi L, Wang Y-H, Yang P-P, Gao F-Y, Wu Z-Z, Chi L-P. Efficient acidic hydrogen evolution in proton exchange membrane electrolyzers over a sulfur-doped marcasite-type electrocatalyst. Sci Adv. 2023;9:eadh2885.
Sun X, Liu F, Chen X, Li C, Yu J, Pan M. Iridium-doped ZIFs-derived porous carbon-coated IrCo alloy as competent bifunctional catalyst for overall water splitting in acid medium. Electrochim Acta. 2019;307:206.
Kuttiyiel KA, Sasaki K, Chen W-F, Su D, Adzic RR. Core–shell, hollow-structured iridium–nickel nitride nanoparticles for the hydrogen evolution reaction. J Mater Chem A. 2014;2:591.
Jiang P, Huang H, Diao J, Gong S, Chen S, Lu J, Wang C, Sun Z, **a G, Yang K. Improving electrocatalytic activity of iridium for hydrogen evolution at high current densities above 1000 mA cm−2. Appl Catal B Environ. 2019;258:117965.
Cai J, Song Y, Zang Y, Niu S, Wu Y, **e Y, Zheng X, Liu Y, Lin Y, Liu X. N-induced lattice contraction generally boosts the hydrogen evolution catalysis of P-rich metal phosphides. Sci Adv. 2020;6:eaaw8113.
Seo H, Cho KH, Ha H, Park S, Hong JS, ** K, Nam KT, Seo H, Cho KH, Ha H. Water oxidation mechanism for 3d transition metal oxide catalysts under neutral condition. J Korean Ceram Soc. 2017;54:1.
Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20.
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys-Condens Matter. 2009;21:395502.
Giannozzi P, Andreussi O, Brumme T, Bunau O, Nardelli MB, Calandra M, Car R, Cavazzoni C, Ceresoli D, Cococcioni M. Advanced capabilities for materials modelling with Quantum ESPRESSO. J Phys-Condens Matter. 2017;29:465901.
Virtual Lab. Inc. MS. 2017. https://www.materialssquare.com.
Ernzerhof M, Perdew JP. Generalized gradient approximation to the angle-and system-averaged exchange hole. J Chem Phys. 1998;109:3313.
Nørskov JK, Bligaard T, Logadottir A, Kitchin J, Chen JG, Pandelov S, Stimming U. Trends in the exchange current for hydrogen evolution. J Electrochem Soc. 2005;152:J23.
Bhattacharjee S, Waghmare UV, Lee S-C. An improved d-band model of the catalytic activity of magnetic transition metal surfaces. Sci Rep. 2016;6:35916.
ElementData, Wolfram Language function, Wolfram Research, https://reference.wolfram.com/language/ref/ElementData.html updated 2014. (2007).
Zhao R, Liu C, Zhang X, Zhu X, Wei P, Ji L, Guo Y, Gao S, Luo Y, Wang Z, Sun X. An ultrasmall Ru2P nanoparticles–reduced graphene oxide hybrid: an efficient electrocatalyst for NH3 synthesis under ambient conditions. J Mater Chem A. 2020;8:77.
Su L, ** Y, Gong D, Ge X, Zhang W, Fan X, Luo W. The role of discrepant reactive intermediates on Ru-Ru2P heterostructure for pH-universal hydrogen oxidation reaction. Angew Chem Int Ed. 2023;135:e202215585.
Liu T, Feng B, Wu X, Niu Y, Hu W, Li CM. Ru2P nanoparticle decorated P/N-doped carbon nanofibers on carbon cloth as a robust hierarchical electrocatalyst with platinum-comparable activity toward hydrogen evolution. ACS Appl Energy Mater. 2018;1:3143.
Vanni M, Provinciali G, Calvo FD, Carignani E, Dreyfuss S, Mézailles N, Mio AM, Nicotra G, Caporali S, Borsacchi S. Ru-P nanoalloy from elemental phosphorus as P-source: synthesis, characterization and catalytic evaluation. ChemCatChem. 2022;14:e202200685.
Yu W-L, Chi J-Q, Dong B. Reduction tuning of ultrathin carbon shell armor covering IrP2 for accelerated hydrogen evolution kinetics with Pt-like performance. J Mater Chem A. 2021;9:2195.
Yang Y, Yang P, Zhou L, He R, Hao Y, Wang J, Qiu R, Zhao X, Yang L. Electrospun IrP2-carbon nanofibers for hydrogen evolution reaction in alkaline medium. Appl Surf Sci. 2021;565:150461.
Pu Z, Zhao J, Amiinu IS, Li W, Wang M, He D, Mu S. A universal synthesis strategy for P-rich noble metal diphosphide-based electrocatalysts for the hydrogen evolution reaction. Energy Environ Sci. 2019;12:952.
Shinagawa T, Garcia-Esparza AT, Takanabe K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci Rep. 2015;5:13801.
Gao Y, Chen Z, Zhao Y, Yu W, Jiang X, He M, Li Z, Ma T, Wu Z, Wang L. Facile synthesis of MoP-Ru2P on porous N, P co-doped carbon for efficiently electrocatalytic hydrogen evolution reaction in full pH range. Appl Catal B Environ. 2022;303:120879.
An L, Bai L, Sun Y, Tang L, Ma L, Guo J, Liu Q, Zhang X. Ru2P particles decorated Ni2P nanosheet as efficient and pH-universal material for hydrogen evolution. Appl Surf Sci. 2020;520:146363.
Miao H, Zhang D, Shi Y, Wu X, Zhang W, Chen X, Lai J, Wang L. Ultrasmall noble metal doped Ru2P@Ru/CNT as high-performance hydrogen evolution catalysts. ACS Sustain Chem Eng. 2021;9:15063.
Li Y, Chu F, Bu Y, Kong Y, Tao Y, Zhou X, Yu H, Yu J, Tang L, Qin Y. Controllable fabrication of uniform ruthenium phosphide nanocrystals for the hydrogen evolution reaction. Chem Commun. 2019;55:7828.
Chang Q, Ma J, Zhu Y, Li Z, Xu D, Duan X, Peng W, Li Y, Zhang G, Zhang F. Controllable synthesis of ruthenium phosphides (RuP and RuP2) for pH-universal hydrogen evolution reaction. ACS Sustain Chem Eng. 2018;6:6388.
Chi J-Q, Gao W-K, Lin J-H, Dong B, Yan K-L, Qin J-F, Liu B, Chai Y-M, Liu C-G. Hydrogen evolution activity of ruthenium phosphides encapsulated in nitrogen- and phosphorous-codoped hollow carbon nanospheres. Chemsuschem. 2018;11:743.
Luo Q, Xu C, Chen Q, Wu J, Wang Y, Zhang Y, Fan G. Synthesis of ultrafine ruthenium phosphide nanoparticles and nitrogen/phosphorus dual-doped carbon hybrids as advanced electrocatalysts for all-pH hydrogen evolution reaction. Int J Hydrogen Energy. 2019;44:25632.
Luo W, Wang Y, Li X, Cheng C. RuP nanoparticles on ordered macroporous hollow nitrogen-doped carbon spheres for efficient hydrogen evolution reaction. Nanotechnology. 2020;31:295401.
Cheng M, Geng H, Yang Y, Zhang Y, Li CC. Optimization of the hydrogen-adsorption free energy of Ru-based catalysts towards high-efficiency hydrogen evolution reaction at all pH. Chem Eur J. 2019;25:8579.
** X, Jang H, Jarulertwathana N, Kim MG, Hwang S-J. Atomically thin holey two-dimensional Ru2P nanosheets for enhanced hydrogen evolution electrocatalysis. ACS Nano. 2022;16:16452.
Han ZJ, Pineda S, Murdock AT, Seo DH, Ostrikov K, Bendavid A. RuO2-coated vertical graphene hybrid electrodes for high-performance solid-state supercapacitors. J Mater Chem A. 2017;5:17293.
Tomikawa K, Kanno H. Raman study of sulfuric acid at low temperatures. J Phys Chem A. 1998;102:6082.
Turner D. Raman spectral study of bisulphate ion hydration. J Chem Soc-Perkin Trans 2. 1972;68:643.
Lund Myhre CE, Christensen DH, Nicolaisen FM, Nielsen CJ. Spectroscopic study of aqueous H2SO4 at different temperatures and compositions: variations in dissociation and optical properties. J Phys Chem A. 1979;2003:107.
Liu T, Wang S, Zhang Q, Chen L, Hu W, Li CM. Ultrasmall Ru2P nanoparticles on graphene: a highly efficient hydrogen evolution reaction electrocatalyst in both acidic and alkaline media. Chem Commun. 2018;54:3343.
Takigawa I, Shimizu K, Tsuda K, Takakusagi S. Machine-learning prediction of the d-band center for metals and bimetals. RSC Adv. 2016;6:52587.
Acknowledgements
This research was supported by (i) the National R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2021M3D1A2051636), and (ii) an NRF funded by the Ministry of Science and ICT (2023M3H4A1A03061436).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by KJ, DSAP, AH, JIJ, HMK, and J-CK. The original draft of the manuscript was written by KJ. Conceptualization, validation, and editing of draft were performed by CWL and D-WK. All authors have commented on previous versions of the manuscript and have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jung, K., Pratama, D.S.A., Haryanto, A. et al. Iridium-Cluster-Implanted Ruthenium Phosphide Electrocatalyst for Hydrogen Evolution Reaction. Adv. Fiber Mater. 6, 158–169 (2024). https://doi.org/10.1007/s42765-023-00342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-023-00342-z