Abstract
Serum uric acid (SUA) has been discovered to be associated with bone mineral density (BMD), but its relationship with trabecular bone score (TBS) remains unclear. Thus, the aim of our study was to investigate the association between SUA levels and TBS. Our study included 5895 individuals over 20 years old (3061 men and 2834 women) from NHANES 2005–2008. To analyze the association between SUA and TBS, multivariate linear regression models with covariate adjustments were applied. Furthermore, population description, stratified analysis, single factor analysis, smooth curve fitting, interaction analysis, and threshold effect and saturation effect analysis were also conducted. After adjusting for covariates, SUA showed a strong negative relationship with total TBS (β = 0.319; 95% CI 0.145–0.494; P < 0.001). The relationship between SUA levels and total TBS was found to be nonlinear, with inflection points at 4.8 mg/dL for the overall population, 4.2 mg/dL for women, and 5.7 mg/dL for non-Hispanic whites, indicating a saturation effect. Additionally, no interactions were found in any of the subgroups. Our study found a negative association between SUA and total TBS in adults. Maintaining SUA at a saturated level can benefit in preventing osteoporosis and fractures.
Similar content being viewed by others
Introduction
Osteoporosis is a chronic systemic bone disease characterized by reduced BMD and destruction of bone structure, which seriously affects physical and mental health1. According to estimates based on US Census data, in 2010, there were 10.2 million elderly individuals affected by osteoporosis, and an additional 43.4 million elderly individuals had low bone density, which placed them in a high-risk category for osteoporotic fractures2. The BMD measurement is commonly used to diagnose osteoporosis. Although BMD can identify many individuals who are at risk, using BMD alone to assess fracture risk may not be sufficient, especially for people with normal or higher BMD levels3. TBS is a novel method to reflect bone quality based on the gray-level texture parameters of the lumbar DXA images4. Compared with BMD, TBS helps detect populations with degraded bone microstructure but normal BMD, and it more accurately predicts osteoporotic fractures5,6. Therefore, TBS, which can reflect bone microstructure and evaluate bone quality, has attracted much attention.
SUA is the end product of purine metabolism in humans. Elevated SUA levels or hyperuricemia can lead to a series of diseases, such as gouty arthritis, diabetes mellitus (DM), chronic kidney disease (CKD), cardiovascular disease, and metabolic syndrome7,8,9,10. Meanwhile, recent studies have found that increased SUA levels may be linked to a decreased prevalence of osteoporosis11. Its mechanism may be related to the antioxidant effect of SUA12. Furthermore, SUA can also affect the level of bone metabolism by regulating the activities of osteoclasts and osteoblasts13,14. However, some studies have not supported the association between uric acid and bone density or osteoporosis15,16,17. Therefore, the effect of SUA remains controversial.
Currently, the relationship between SUA and TBS is seldom reported. To address this, we collected data from the National Health and Nutrition Examination Survey (NHANES) from 2005 to 2008, through strict inclusion criteria and covariate adjustments, to clarify the relationship between SUA and total TBS.
Method
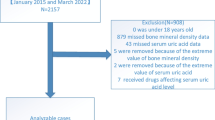
NHANES is a nationally representative cross-sectional survey aimed at providing abundant information on the overall health and nutritional status of the United States (US) population18. Detailed data on NHANES can be found on the Internet and has been approved by the National Center for Health Statistics (NCHS) Ethics Review Board19. For this cross-sectional study, we collected 20,974 individuals from NHANES 2005–2008 and ultimately identified 5895 participants through strict inclusion criteria. The specific process is shown in Fig. 1. Exclusion criteria included: age < 20 years old; missing SUA and total TBS data; diabetes mellitus; rheumatoid arthritis; chronic kidney disease (estimated glomerular filtration rate [eGFR < 60 mL/min/1.73 m2]); cancer; or participants using diphosphonate, glucocorticoids, estrogen, diuretics, allopurinol therapy. Finally, out of 20,497 participants, a total of 5895 eligible individuals without serious illnesses were included in the study.
The study measured the independent variable, SUA, using the Beckman Synchron LX20. The dependent variable was total TBS. It was considered to be a textural index and measured by evaluating gray-level variations of lumbar spine DXA scanning pixels. The spine scans were obtained using Hologic QDR-4500A fan-beam densitometers (Hologic, Inc., Bedford, Massachusetts). Subsequently, the total TBS score was estimated in adults aged 20 years and older through TBS software (Med-Imap SA TBS Calculator version 2.1.0.2). Meanwhile, for statistical analysis, quartiles of SUA levels were divided into four groups: the first quartile (Q1): 0.5–4.4 mg/dL, the second quartile (Q2): 4.5–5.3 mg/dL, the third quartile (Q3): 5.4–6.2 mg/dL, and the fourth quartile (Q4): 6.3–11 mg/dL.
Based on prior research and clinical experience, we incorporated the subsequent covariates that might influence the association between SUA and TBS. The continuous covariates in this study were age, height, weight, body mass index (BMI), waist circumference, alanine transaminase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), total protein, cholesterol, high-density lipoprotein cholesterol (HDL-C), serum phosphorus, blood urea nitrogen, serum calcium, serum albumin, serum creatinine, C-reactive protein (CRP), total femur bone mineral density (BMD), total spine bone mineral density (BMD), calcium supplementation, and estimated glomerular filtration rate (eGFR). The categorical variables among the covariates were gender, age, BMI, race/ethnicity, education level, marital status, income to poverty ratio, drinking status (Had at least 12 alcohol drinks a year?), sleep, smoke. Details of each variable are available on the NHANES website. The poverty income ratio is determined by dividing family income by the poverty guidelines set by the Department of Health and Human Services (HHS). This ratio is used to measure poverty and is categorized as "mild" (poverty income ratio < 1.99), "moderate" (1.99 ≤ poverty income ratio ≤ 3.49), or "severe" (poverty income ratio > 3.49). The equation for estimating GFR20 is shown in Supplementary Table S1.
Statistical analyses
Before conducting the data analysis for this study, we performed normality tests on all variables using histograms and P-P plots. The comparison of continuous variables was done using a weighted linear regression model, while the comparison of categorical variables was done using a weighted chi-square test. The final analysis was expressed as mean ± standard deviation (SD; continuous variable) or percentages (categorical variable). In three different models, weighted multivariate linear regression analysis was used to evaluate the correlation between SUA and total TBS. The three models are as follows: Model 1, a non-adjusted model; Model 2, minimally adjusted for gender, age, and race/ethnicity; Model 3, fully adjusted for all covariates. We also conducted group analysis and performed interaction tests on subgroups. Furthermore, the generalized additive models (GAM) and smooth curve fittings were further applied in the fully adjusted model to check the nonlinear or linear correlation between SUA and total TBS. Determine whether there is a non-linear relationship based on the log-likelihood ratio. If there is a non-linear relationship, a two-stage linear regression model is used to calculate the inflection point of SUA on total TBS using a recursive algorithm.
When the p-value is less than 0.05 (two-sided), it has statistical significance. All statistical analyses were performed using EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA) and R statistics packages (http://www.R-project.org, The R Foundation).
Ethics statement
According to local laws and institutional requirements, this study did not require ethical review and approval. Participants provided written informed consent to participate in this study.
Results
Characteristics of the study population
The study involved a total of 5895 participants aged 20 years or older, with an average age of 44.66 ± 16.00 years. Weighted demographics and clinical characteristics according to the quartile of SUA were shown in Table 1. The missing data for covariates are shown in Supplementary Table S2. Missing data for categorical variables were treated as "Not recorded" categories; missing data for continuous variables were coded as mean values. There were significant differences in baseline characteristics of the SUA quartiles except for income to poverty ratio and race/ethnicity. Men, more than high school, married/living with partner, drinkers, and sleep time (7–8 h) had higher levels of SUA in the top quartile (Q4). Interestingly, women may have lower levels of SUA in the quartiles (Q1 and Q2).
Univariate analysis
In weighted univariate analysis (Supplementary Table S3), some variables have a significant correlation with total TBS, including age, women, marital status, more than high school, weight, height, BMI, income to poverty ratio (High), non-drinkers, sleep, waist circumference, calcium supplementation, serum creatinine, eGFR, ALT, AST, ALP, serum albumin, blood urea nitrogen, serum calcium, cholesterol, serum phosphorus, CRP, total femur BMD, total spine BMD, and SUA. However, no significant correlation was found in the remaining variables.
Association between SUA and total TBS
As shown in Table 2, significant negative correlations were observed between SUA and total TBS in all three multivariate linear regression models: Model 1 (β = − 0.0247, 95% CI − 0.0273, − 0.0222); Model 2 (β = − 0.0257, 95% CI − 0.0284, − 0.0230); Model 3 (β = − 0.0036, 95% CI − 0.0057, − 0.0015). In the fully adjusted model (Model 3), for every additional unit of SUA, TBS decreases by 0.0036. After converting SUA to categorical variable (quartile), the SUA of the highest quartile was 0.0120 mg/dL lower than the lowest quartile. At the same time, P for trend test all had P < 0.001, indicating that the downward trend of TBS was significant with the increase of SUA level.
Figure 2 reflects the results of subgroup analysis and the interaction analysis of the association between SUA and total TBS. In all subgroup analyses, there was no significant correlation between SUA and total TBS among men, adults ≥ 60 years or < 40 years, Mexican Americans, never married, sleep duration (< 6 h), and less than high school. Simultaneously, there was no interaction observed in any of the subgroups (P for interaction > 0.05).
In generalized additive models and smooth curve fittings, the nonlinear relationship and saturation effect between SUA and total TBS have been found (Fig. 3). In addition, we also found that the saturation effect value between the SUA and total TBS was 4.8 mg/dL by using a two-piecewise linear regression model (Table 3). For a SUA < 4.8 mg/dL, every 1 mg/dL increase in SUA was associated with a 0.003 greater total TBS (95% CI − 0.004–0.031, p = 0.1779). By contrast, for participants with a SUA > 4.8 mg/dL, a 1 mg/dL increase in SUA was associated with a decrease of 0.006 in total TBS (95% CI − 0.009 to − 0.004, p < 0.0001), which was statistically significant. We also examined the relationship between SUA levels and total TBS stratified by gender, age, and race/ethnicity (Fig. 4). Among women, Mexican Americans and non-Hispanic white, the relationship between SUA and total TBS was an inverted U-shaped curve, with inflection points of 4.3 mg/dL, 5.9 mg/dL and 5.7 mg/dL, respectively (Supplementary Tables S4–S6). And there are multiple inflection points among non-Hispanic black, at 4.9 mg/dL and 6.9 mg/dL (Supplementary Table S7). However, in stratified analysis, there was no statistical significance among the Mexican American population.
Discussion
In this cross-sectional study, nationally representative data from the US population aged 20 and above from 2015 to 2018 were used to evaluate the association between SUA levels and total TBS. The results showed a significant negative correlation between SUA and total TBS. The association between SUA and total TBS was not significant in men, adults aged ≥ 60 or < 40, Mexican Americans, never married, sleep duration (< 6 h), and less than high school. Additionally, we also observed a nonlinear relationship between SUA and total TBS, presenting an inverted U-shaped inflection point at 4.8 mg/dL. This inverted U-shaped association also exists among women and non-Hispanic white populations. From this, it can be inferred that controlling SUA within a reasonable range can achieve better TBS, which may help prevent osteoporosis and osteoporotic fractures.
With the rapid development of aging population, osteoporosis has become one of the most common threats to the safety of public healthcare. The final result often leads to many adverse consequences such as fractures. As is well known, BMD measurement based on DXA is an important method for diagnosing osteoporosis. However, it can only reflect bone content data and cannot provide information on bone quality21. For most patients with brittle fractures, the T-value is often within the range of low or even normal bone mass. In contrast, the TBS is a novel method for assessing skeletal microstructure from two-dimensional DXA images, which correlates directly with the mechanical strength of the bone22,23. Compared to BMD, TBS can be a tool for obtaining more comprehensive skeletal data, hel** to detect individuals with microstructure degradation but normal bone density4,22. Higher TBS results correspond to better bone structure, while lower TBS results reflect the worse bone structure and higher fracture risk24. Research shows that the combination of the Fracture Risk Assessment Tool (FRAX) score and TBS can enhance the accuracy of fracture risk prediction25,26. Furthermore, the ability of TBS to predict fracture was not affected by BMD and most clinical risk factors5,27. Reviewing recent studies, numerous factors have been shown to be associated with TBS, such as obesity, sleep duration, and diabetes28,29,30. Therefore, more and more people pay attention to the related research of TBS. However, the association between serum SUA and TBS is still unclear.
Recent research reports suggest that SUA may play a beneficial role in certain diseases, such as osteoporosis31. This may be related to the antioxidant effect of uric acid, which can prevent oxidative stress (OS) related bone loss and osteoporosis32. OS can alter the process of bone remodeling by affecting the activity of osteoclasts and osteoblasts, and increase bone turnover rate, ultimately leading to osteoporosis14,33,34. Additionally, OS affects the proliferation of bone marrow mesenchymal stromal cells and osteoblast precursors35,36. Research datas show that natural antioxidants can prevent or reverse the negative effects of OS on the bone tissue by maintaining bone cell activity, activating osteoblast differentiation, and mineralization processes37,38. In a large cross-sectional study, a positive correlation was found between higher SUA and greater BMD39,40, which was supported by other Asian studies41,42. Similarly, a study of 17,329 participants from South Korea revealed that hyperuricemia was linked to a decreased risk of osteoporosis43. In addition, SUA also has the effect of preventing free radicals from damaging blood vessels, heart, and neurons12,44. However, the antioxidant properties of SUA may be influenced by the hydrophobic lipid layer of the cell membrane45. Meanwhile, SUA degradation may also produce intracellular free oxygen radicals and interact with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to enhance intracellular superoxide46. This result will inhibit osteoblast bone formation and stimulate osteoclast bone absorption.
Although SUA may have contradictory effects on bone metabolism, our study further indicates a significant negative correlation between SUA levels and total TBS. Whether the covariates are adjusted or not, this result is statistically significant. In the subgroup analysis, this negative correlation still exists, especially among women aged 40 to 60 (≥ 40, < 60) with a high school education or above, excluding Mexican Americans. We also found that there is a generally lower total TBS value in elderly patients (≥ 60). Maintaining uric acid at an appropriate level to obtain a high total TBS value helps to further prevent the occurrence of osteoporotic fractures and provides guidance for clinical practice.
In this study, some advantages are worth noting. First of all, this is the first study to explore the correlation between SUA levels and total TBS. Next, this study used large sample data and a multivariate adjusted model to control for related confounding factors. In addition, we conducted stratified analysis, generalized additive model, and smooth curve fitting analysis to discover the multifaceted effects of SUA levels on total TBS. Of course, this study also has its limitations. Firstly, this study is a cross-sectional study and cannot determine the causal relationship between SUA levels on total TBS. Secondly, the research subjects are mainly American participants, and it is not yet known whether they are applicable to other regions or countries. Furthermore, variations in dietary and lifestyle habits may also influence uric acid levels47,48.
Conclusion
In the adult population of the US, our study found a significant negative correlation between SUA levels and total TBS, which follows an inverted U-shaped curve (inflection point: 4.8 mg/dL). Meanwhile, this inverted U-shaped curve also exists among non-Hispanic white women. This study suggests that maintaining SUA at a saturation level can provide the optimal total TBS value for adults and may help prevent osteoporosis and osteoporotic fractures.
Data availability
The data that support the results of this study can obtained from https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. The availability of this data is not restricted.
References
Ensrud, K. E. & Crandall, C. J. Osteoporosis [published correction appears in Ann Intern Med. 2017;167(7):528]. Ann. Intern. Med. 167(3), 17–32. https://doi.org/10.7326/AITC201708010 (2017).
Wright, N. C. et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 29(11), 2520–2526. https://doi.org/10.1002/jbmr.2269 (2014).
Wainwright, S. A. et al. Hip fracture in women without osteoporosis. J. Clin. Endocrinol. Metab. 90(5), 2787–2793. https://doi.org/10.1210/jc.2004-1568 (2005).
Silva, B. C. et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image [published correction appears in J Bone Miner Res. 2017;32(11):2319]. J. Bone Miner. Res. 29(3), 518–530. https://doi.org/10.1002/jbmr.2176 (2014).
Hans, D., Goertzen, A. L., Krieg, M. A. & Leslie, W. D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J. Bone Miner. Res. 26(11), 2762–2769. https://doi.org/10.1002/jbmr.499 (2011).
McCloskey, E. V. et al. Adjusting fracture probability by trabecular bone score. Calcif. Tissue Int. 96(6), 500–509. https://doi.org/10.1007/s00223-015-9980-x (2015).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N. Engl. J. Med. 359(17), 1811–1821. https://doi.org/10.1056/NEJMra0800885 (2008) (Erratum in: N Engl J Med. 362:2235).
Oda, E., Kawai, R., Sukumaran, V. & Watanabe, K. Uric acid is positively associated with metabolic syndrome but negatively associated with diabete-s in Japanese men. Intern. Med. 48(20), 1785–1791. https://doi.org/10.2169/internalmedicine.48.2426 (2009).
Srivastava, A., Kaze, A. D., McMullan, C. J., Isakova, T. & Waikar, S. S. Uric acid and the risks of kidney failure and death in individuals with CKD. Am. J. Kidney Dis. 71(3), 362–370. https://doi.org/10.1053/j.ajkd.2017.08.017 (2018).
Dalbeth, N. et al. Gout. Nat. Rev. Dis. Primers. 5(1), 69. https://doi.org/10.1038/s41572-019-0115-y (2019).
Yan, D. D. et al. Association of serum uric acid levels with osteoporosis and bone turnover m-arkers in a Chinese population. Acta Pharmacol. Sin. 39(4), 626–632. https://doi.org/10.1038/aps.2017.165 (2018).
Glantzounis, G. K., Tsimoyiannis, E. C., Kappas, A. M. & Galaris, D. A. Uric acid and oxidative stress. Curr. Pharm. Des. 11(32), 4145–4151. https://doi.org/10.2174/138161205774913255 (2005).
Agidigbi, T. S. & Kim, C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int. J. Mol. Sci. 20(14), 3576. https://doi.org/10.3390/ijms20143576 (2019).
Domazetovic, V., Marcucci, G., Iantomasi, T., Brandi, M. L. & Vincenzini, M. T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 14(2), 209–216. https://doi.org/10.11138/ccmbm/2017.14.1.209 (2017).
Dalbeth, N. et al. Elevated urate levels do not alter bone turnover markers: Randomized controlled trial of inosine supplementation in postmenopausal women. Arthritis Rheumatol. 73(9), 1758–1764. https://doi.org/10.1002/art.41691 (2021).
Hu, Z. et al. Prevalence and risk factors for bone loss in rheumatoid arthritis patients from South China: Modeled by three methods. BMC Musculoskelet. Disord. 22(1), 534. https://doi.org/10.1186/s12891-021-04403-5 (2021).
Li, X., Li, L., Yang, L., Yang, J. & Lu, H. No association between serum uric acid and lumbar spine bone mineral density in US adult males: A cross sectional study. Sci. Rep. 11(1), 15588. https://doi.org/10.1038/s41598-021-95207-z (2021).
Curtin, L. R. et al. The national health and nutrition examination survey: Sample design, 1999–2006. Vital Health Stat. 155, 1–39 (2012).
Zipf, G. et al. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. 56, 1–37 (2013).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Dalle Carbonare, L. & Giannini, S. Bone microarchitecture as an important determinant of bone strength. J. Endocrinol. Invest. 27(1), 99–105. https://doi.org/10.1007/BF03350919 (2004).
Harvey, N. C. et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 78, 216–224. https://doi.org/10.1016/j.bone.2015.05.016 (2015).
Pothuaud, L., Carceller, P. & Hans, D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: Applications in the study of human trabecular bone microarchitecture. Bone. 42(4), 775–787. https://doi.org/10.1016/j.bone.2007.11.018 (2008).
Ulivieri, F. M. et al. Utility of the trabecular bone score (TBS) in secondary osteoporosi-s. Endocrine. 47(2), 435–448. https://doi.org/10.1007/s12020-014-0280-4.cvu (2014).
McCloskey, E. V. et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Miner. Res. 31(5), 940–948. https://doi.org/10.1002/jbmr.2734 (2016).
Hans, D., Šteňová, E. & Lamy, O. The trabecular bone score (TBS) complements DXA and the FRAX as a fracture risk assessment tool in routine clinical practice. Curr. Osteoporos. Rep. 15(6), 521–531. https://doi.org/10.1007/s11914-017-0410-z (2017).
Martineau, P. et al. Clinical utility of using lumbar spine trabecular bone score to adjust fracture probability: The Manitoba BMD cohort. J. Bone Miner. Res. 32(7), 1568–1574. https://doi.org/10.1002/jbmr.3124 (2017).
Langsetmo, L. et al. The association between trabecular bone score and lumbar spine volumetric BMD is attenuated among older men with high body mass index. J. Bone Miner. Res. 31(10), 1820–1826. https://doi.org/10.1002/jbmr.2867 (2016).
Shiao, Y. C., Chen, W. T. & Chen, W. L. Association of short sleep duration and trabecular bone score. Sci. Rep. 11(1), 19821. https://doi.org/10.1038/s41598-021-99410-w (2021).
Holloway, K. L. et al. Trabecular bone score in men and women with impaired fasting glucose and diabetes. Calcif. Tissue Int. 102(1), 32–40. https://doi.org/10.1007/s00223-017-0330-z (2018).
Kaushal, N., Vohora, D., Jalali, R. K. & Jha, S. Review of the literature examining the association of serum uric acid with osteoporosis and mechanistic insights into its effect on bone metabolism. Endocr. Metab. Immune Disord. Drug Targets. 19(3), 259–273. https://doi.org/10.2174/1871530318666181102115106 (2019).
Fabbrini, E., Serafini, M., Colic Baric, I., Hazen, S. L. & Klein, S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 63(3), 976–981. https://doi.org/10.2337/db13-1396 (2014).
Bonaccorsi, G., Piva, I., Greco, P. & Cervellati, C. Oxidative stress as a possible pathogenic cofactor of post-menopausal osteoporosis: Existing evidence in support of the axis oestrogen deficiency-redox imbalance-bone loss. Indian J. Med. Res. 147(4), 341–351. https://doi.org/10.4103/ijmr.IJMR_524_18 (2018).
Mohamad, N. V., Ima-Nirwana, S. & Chin, K. Y. Are oxidative stress and inflammation mediators of bone loss due to estrogen deficiency? A review of current evidence. Endocr. Metab. Immune Disord. Drug Targets. 20(9), 1478–1487. https://doi.org/10.2174/1871530320666200604160614 (2020).
Domazetovic, V. et al. Blueberry juice protects osteocytes and bone precursor cells against oxidativestress partly through SIRT1. FEBS Open Bio. 9(6), 1082–1096. https://doi.org/10.1002/2211-5463.12634 (2019).
Oryan, A., Kamali, A., Moshiri, A. & Baghaban, E. M. Role of mesenchymal stem cells in bone regenerative medicine: What is the evidence?. Cells Tissues Organs. 204(2), 59–83. https://doi.org/10.1159/000469704 (2017).
Austermann, K., Baecker, N., Stehle, P. & Heer, M. Putative effects of nutritive polyphenols on bone metabolism in vivo-evidence from human studies. Nutrients. 11(4), 871. https://doi.org/10.3390/nu11040871 (2019).
Domazetovic, V. et al. Blueberry juice antioxidants protect osteogenic activity against oxidative stress and improve long-term activation of the mineralization process in human osteoblast-like SaOS-2 cells: Involvement of SIRT1. Antioxidants 9(2), 125. https://doi.org/10.3390/antiox9020125 (2020).
Dong, X. W. et al. Elevated serum uric acid is associated with greater bone mineral density and skeletal muscle mass in middle-aged and older adults. PLoS ONE. 11(5), e0154692. https://doi.org/10.1371/journal.pone.0154692 (2016).
Chen, F. et al. Specific higher levels of serum uric acid might have a protective effect on bone mineral density within a Chinese population over 60 years old: A cross-sectional study from northeast China. Clin. Interv. Aging. 14, 1065–1073. https://doi.org/10.2147/CIA.S186500 (2019).
Babaei, M., Shamsi, R., Heidari, B., Bijani, A. & Hosseini, S. R. Serum uric acid status and its association with bone mineral density in the elderly people aged 60 years and more. Int. J. Endocrinol. Metab. 17(3), e80780. https://doi.org/10.5812/ijem.80780 (2019).
Lee, Y. J. et al. The association between oxidative stress and bone mineral density according to menopausal status of Korean women. Obstet. Gynecol. Sci. 58(1), 46–52. https://doi.org/10.5468/ogs.2015.58.1.46 (2015).
Lee, J. W., Kwon, B. C. & Choi, H. G. Analyses of the relationship between hyperuricemia and osteoporosis. Sci. Rep. 11(1), 12080. https://doi.org/10.1038/s41598-021-91570-z (2021).
Stocker, R. & Keaney, J. F. Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84(4), 1381–1478. https://doi.org/10.1152/physrev.00047.2003 (2004).
Sautin, Y. Y. & Johnson, R. J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 27(6), 608–619. https://doi.org/10.1080/15257770802138558 (2008).
Steinbeck, M. J., Appel, W. H. Jr., Verhoeven, A. J. & Karnovsky, M. J. NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J. Cell Biol. 126(3), 765–772. https://doi.org/10.1083/jcb.126.3.765 (1994).
Lee, S. J., Terkeltaub, R. A. & Kavanaugh, A. Recent developments in diet and gout. Curr. Opin. Rheumatol. 18(2), 193–198. https://doi.org/10.1097/01.bor.0000209434.82096.1f (2006).
Ebrahimpour-Koujan, S., Saneei, P., Larijani, B. & Esmaillzadeh, A. Consu-mption of sugar sweetened beverages and dietary fructose in relation to risk of gout and hyperuricemia: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 60(1), 1–10. https://doi.org/10.1080/10408398.2018.1503155 (2020).
Funding
This study was supported by grants from the Department of Health of Zhejiang Province (2020KY408 to Dr. Jun Zhang, 2022KY548 to Dr. Haiyu Shao, and 2022KY608 to Dr. Tingxiao Zhao).
Author information
Authors and Affiliations
Contributions
Y.L., J.T., J.T. contributed to the conception and wrote the manuscript of the study; J.X., H.S., J.Z. were responsible for data collection and processing; T.Z., Y.H. performed the data analyses and constructive discussions. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Tan, J., Tian, J. et al. Cross-sectional analysis of the correlation between serum uric acid and trabecular bone score: NHANES 2005–2008. Sci Rep 13, 21546 (2023). https://doi.org/10.1038/s41598-023-48739-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48739-5
- Springer Nature Limited