Abstract
Electrolytic water hydrogen production systems are highly coupled and operate under complex and dangerous conditions, making it difficult to carry out extreme experiments on physical objects. In this paper, the alkaline electrolyzer is studied and mathematical and three-dimensional models are developed for it. The System operation data shows that temperature, pressure and alkaline flow rate all have effects on the hydrogen concentration in oxygen and voltage. Among them, hydrogen concentration in oxygen is one of the important factors affecting the safety of the system, and changing both pressure and alkali flow rate respectively will cause significant changes in hydrogen concentration. Based on the system operation data, a multivariate nonlinear fitting of the empirical equation using Matlab was performed to establish the voltage model, Faraday efficiency model, hydrogen concentration in oxygen model and system pressure model of the alkaline electrolyzer. And the model simulation results were analyzed and verified theoretically, which were consistent with the actual engineering. The mathematical model of the alkaline electrolyzer is combined with the 3D model, and the digital twin model of the alkaline electrolyzer is controlled in real time by PLC. The map** of the alkaline electrolyzer in the virtual space is realized, which provides an experimental platform for the subsequent study of wide power fluctuation.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
As the “ultimate energy source for mankind”, hydrogen energy has the advantages of high calorific value, recyclability, cleanliness and non-pollution, which have strengthened the position of hydrogen energy in the future energy system in various countries [7] found that the system pressure can affect the hydrogen content in oxygen by experimenting with a 250 kW electrolyzer, and the electrolyzer can be operated stably at a wide power range of 30–100% by adjusting the system pressure, but they did not compare the effects of other process parameters. Ning et al. [8] conducted an experimental study on the electrolyzer with wide power from the perspective of safety and showed that proper adjustment of lye flow could stabilize the hydrogen content in oxygen within the safety range, but the system pressure was not considered. In order to study the control method of wide power, the establishment of mathematical model of the electrolyzer is particularly important. Fang et al. [9] proposed and simulated a control strategy for a modular switching electrolyzer based on the empirical equation given by Ulleberg [10], and the simulation results showed that this control strategy improved the hydrogen production rate. Ulleberg [10] established a thermal model and a mathematical model of the alkaline electrolyzer based on thermodynamics, heat transfer and electrochemical theory, and this model is applicable and has been widely used in the study of mathematical models of electrolytic cells. However, Ulleberg only considered the effect of temperature and ignored the effect of other factors on the electrolyzer. Mónica et al. [12] added the effect of system pressure to Ulleberg’s model and developed a semi-empirical mathematical model for a 15KW electrolyzer, but this literature did not consider the alkaline flow rate, which was shown to have an effect on the hydrogen content in oxygen [8, 14]. Jiang et al. [11] developed a digital twin model of an alkaline electrolyzer based on experiments and principles with temperature as a variable, but did not visualize and control the model results in real time.
In this paper, the alkaline electrolyzer is studied and its mathematical model and three-dimensional model are established. The mathematical model includes voltage model, Faraday efficiency model, hydrogen concentration in oxygen model and system pressure model. The voltage model and the hydrogen concentration in oxygen model not only consider the pressure and temperature, but also take into account the alkali flow rate which has a great influence on both of them; the alkali flow rate is also used as one of the variables in the Faraday efficiency model, but the influence is very small. In this paper, a three-dimensional model of the hydrogen production system is also developed, since few studies have shown the mathematical model more visually and have controlled and tested it in a simple way. Therefore, a more accurate mathematical model of the alkaline electrolyzer is developed, simulated and validated, and finally combined with the 3D model to form a digital twin model of the alkaline electrolyzer.
2 Alkaline Electrolyzer Digital Twin Model Framework
The digital twin model of the alkaline electrolyzer will be divided into a physical object layer, a data acquisition and control entity layer, an alkaline electrolyzer digital twin core entity layer, and an alkaline electrolyzer digital twin model application layer. The layers are interconnected with each other and progress towards the electrolyzer digital twin model layer by layer. The framework of the digital twin model of the alkaline electrolyzer is shown in Fig. 1.
2.1 Alkaline Electrolyzer Digital Twin Model Hierarchy
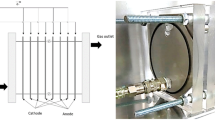
The physical object layer is the physical object of the alkaline electrolytic cell. The hydrogen production system of electrolytic water studied in this paper mainly includes alkaline electrolytic cell, gas-liquid separation and post-processing system. The process diagram of the hydrogen production system of electrolytic water is shown in Fig. 2. The power supply supplies direct current to the electrolytic cell, and the gas produced by the alkaline electrolytic cell flows to the gas separator and scrubber through the alkaline liquid heat exchanger. The lye brought out by the gas is returned to the electrolytic cell through the lye filter under the action of the lye circulating pump. The data acquisition and control entity layer is the various sensors and control flow in the hydrogen production system of electrolytic water. This paper focuses on the core entity layer of digital twins. The established mathematical model is tested in the virtual simulation platform, and the model is controlled and verified by PLC. Finally, the running data are transmitted to the three-dimensional model in real time to realize the construction of the digital twin application layer of the hydrogen production system.
2.2 Mathematical Modeling Framework for Alkaline Electrolyzer
The mathematical modeling process of alkaline electrolyzer integrates the methods of mechanism modeling and data modeling, updates the empirical equation based on experimental data and theoretical analysis, and then uses the operational data of the hydrogen production system to fit the empirical equation nonlinearly, and finally establishes the mathematical model of alkaline electrolyzer.
The mechanism modeling is based on the operation mechanism of the alkaline electrolyzer, the derivation and demonstration of the equations using the knowledge in the field of electrochemistry. The final mathematical model is suitable for the studied alkaline electrolyzer.
3 Alkaline Electrolyzer Digital Twin Model Construction
3.1 Alkaline Electrolyzer Mathematical Model Construction
Modeling of alkaline electrolyzer voltage characteristics
The most commonly used voltage model was proposed in 2003 by Ulleberg [10], who derived an empirical equation for the voltage model of alkaline electrolyzers based on thermodynamics and electrochemical properties of alkaline electrolyzers, as shown in Eq. (1):
where Urev is the reversible voltage, T is the bath temperature, I is the given current, and A is the area of the electrode. s is a specific factor, related to the type and power of the electrolytic bath.
Ernesto Amores et al. [12] based on experiments with a 15KW alkaline electrolyzer, proposed that the voltage model should incorporate the effect of system pressure, but still does not consider the effect of lye flow rate on voltage. The magnitude of the flow rate directly affects the conductivity of the lye and thus the voltage of the alkaline electrolyzer. Therefore, the voltage model of the alkaline electrolyzer should include the effect of the lye flow rate, as in Eq. (2):
In Eq. (2):
In Eq. (5), v is the alkaline flow rate, and including the alkaline flow rate as a variable in the model will make the voltage model of the alkaline electrolyzer more accurate.
Equation (6) is the voltage of each cell, and the total voltage of the alkaline electrolyzer is the number of cells multiplied by the cell voltage.
Modeling of Faraday efficiency characteristics of alkaline electrolyzer
In electrolytic water hydrogen production systems, the amount of hydrogen produced per unit time is closely related to the amount of electricity consumed by the alkaline electrolyzer per unit time, and the Faraday efficiency is defined as the ratio of the actual hydrogen produced by the system per unit time to the theoretical amount of hydrogen produced per unit time, which can be expressed by an empirical equation, derived by Ulleberg et al. [10]:
To take the role of temperature and flow rate into account, Eq. (8) is modified by updating \(f_{1} ,\,f_{2}\) as a function of temperature and flow rate as variables:
Modeling of hydrogen concentration characteristics in oxygen in alkaline electrolyzer
In this paper, we address the modeling of hydrogen concentration in oxygen. According to the previous discussion, the hydrogen content in oxygen can be modeled theoretically based on temperature, pressure, and lye flow rate, etc. Hug et al. [13] in 1993 gave an empirical formula for calculating the hydrogen concentration in oxygen:
Mónica et al. [12] adds the effect of system pressure, but still does not take into account the effect of alkali flow, this paper adds the effect of alkali flow on the basis of Eqs. (11), (12) makes the calculation of hydrogen concentration in oxygen more accurate.
3.2 Alkaline Electrolyzer 3D Model Construction
In this paper, we firstly established a 3D model of the hydrogen production system by using Sketch Up, and then imported the established 3D model into the corresponding topology editor. Based on the established 3D model, this paper uses HT for Web to bring the 3D model closer to the physical entity, mainly by using WebGL and other technologies to visualize the flow of liquid in the system and the circulation pump operation and other physical movements, as shown in Fig. 3.
4 Simulation Study of Digital Twin Model of Alkaline Electrolyzer
In this paper, the parameters of the constructed mathematical model of the alkaline electrolyzer were determined based on the single-day operating data of the 1MW alkaline electrolyzer in Chongli, Zhangjiakou, by using Matlab to perform multiple nonlinear regression on the model. The fitting results are also elaborated and analyzed. The parameters of the alkaline electrolyzer are shown in Table 1.
4.1 Alkaline Electrolyzer Voltage Model Simulation Study
The simulation of the voltage model is carried out according to Eq. (6), and there are four parameters in the updated voltage model, so two of them are fixed separately, and the surface plot of the voltage model is drawn with the other two parameters as variables. As shown in Fig. 4, this paper analyzes the factors affecting the alkaline electrolyzer voltage with (temperature-current density), (pressure-current density), and (lye flow-current density) as variables, respectively.
Figure 5 shows more clearly the effect of temperature, pressure and flow on the cell voltage, from which it can be seen that temperature has the most dramatic effect on pressure, which is the reason why the literature [10] only uses temperature as the only variable in the cell voltage model. In Fig. 5(a, b, c) are taken for different temperature, pressure and flow rate to observe the variation of the cell voltage. Where Fig. 5(c) plots the cell voltage for different lye flow rates, it can be seen that there is a slight decrease in voltage as the lye flow rate increases. The increase in the lye flow rate leads to an increase in the amount of lye cooled per unit time, which leads to a decrease in the lye temperature. The voltage tends to increase as the temperature decreases, which is also shown in Fig. 4(a). On the other hand, the increase in the flow of lye will take away the air bubbles adhering to the electrode in time, which will lower the resistance and therefore the voltage of the cell. It can be seen that the latter cause has a greater effect on the voltage than the former one, because the change in resistance directly affects the voltage, and therefore it will present a slight decrease in voltage.
4.2 Simulation Study of Faraday Efficiency Model for Alkaline Electrolyzer
The simulation study of Faraday efficiency model was carried out according to Eqs. (8), (9) and (10), and the Faraday efficiency of alkaline electrolyzer was plotted with (temperature-current density) and (alkali flow-current density) as variables, respectively, as shown in Fig. 6.
In Fig. 7(a), since an increase in temperature leads to a faster movement of the anions and cations in the alkali solution, the resistance of the electrolyte decreases, which can lead to a small amount of current not being used to produce hydrogen and therefore leads to a decrease in Faraday efficiency. In Fig. 7(b), it can be seen that the effect of alkali flow rate on Faraday efficiency is not very large. It was stated earlier that an increase in the lye flow rate leads to a decrease in temperature, but it also leads directly to a decrease in resistance, a cause also explained in the voltage model, and these two causes have opposite effects on the Faraday efficiency of similar order of magnitude. Therefore, the effect of the lye flow rate on the Faraday efficiency is weak.
4.3 Model Simulation Study of Hydrogen Content in Oxygen
According to the mathematical model of hydrogen concentration in oxygen established in this paper, such as Eq. (12), the variation law of hydrogen concentration in oxygen is plotted with temperature, pressure, and lye flow as variables, as shown in Fig. 8(a), (b), and (c) in Fig. 9 respectively plot the curves of the hydrogen concentration in oxygen varying with the current density under different temperatures, pressures, and lye flow rates. In Fig. 9(a), the current is fixed, and as the temperature increases, the concentration of hydrogen in oxygen increases, and the increase in temperature causes the hydrogen dissolved in the alkali solution to escape and the thermal motion of the gas molecules to accelerate, thereby increasing the diffusion rate. An increase in pressure will lead to an increase in the solubility of the gas, but it will also increase the diffusion rate of gas molecules, as shown in Fig. 9(b). The final result is that the increase in pressure will increase the hydrogen content in oxygen. The lye circulates between the electrolytic cell and the separator under the action of the lye circulation pump. If the flow rate of the lye increases, the amount of lye returned to the hydrogen-oxygen separator per unit time will also increase. Moreover, the high permeability of hydrogen will cause a small amount of hydrogen to dissolve in the lye, which will lead to an increase in the concentration of hydrogen in oxygen, as shown in Fig. 9(c).
5 Conclusions
Based on the data and mechanism, this paper constructs a digital twin model of a 1MW alkaline electrolyzer, including a voltage model, a Faraday efficiency model, a hydrogen concentration model in oxygen, and a system pressure model. The construction of the model is based on data and mathematical formulas, and the simulation results are consistent with the theoretical analysis.
In this paper, we use digital twin technology to simulate the operating characteristics of the alkaline electrolyzer to ensure the safety of the real system operation. The operation characteristics of the hydrogen production system are complex, and the mathematical models of the valve separator and compressor have not been completed, and the digital twin model of the whole hydrogen production system is yet to be solved.
References
Cheng, W.J., Zhao, L., **, H., et al.: Research on hydrogen energy policy and water-electrolytic hydrogen under the 14th Five Year Plan. Therm. Power Gener. 51(11), 181–188 (2022)
Peng, Y., Bai, X.M.: Cities leading hydrogen energy development: the pledges and strategies of 39 Chinese cities. NPJ Urban Sustain. 2(1) (2022)
Acharya, A.: Scaling-up green hydrogen development with effective policy interventions. J. Sustain. Dev. 15(5), 135 (2022)
Wang, P.C., Wan, L., Xu, Q., et al.: Hydrogen production based-on anion exchange membrane water electrolysis: a critical review and perspective. CIESC J. 72(12), 6161–6175 (2021)
Zheng, Y., You, S., Bindner, H.W., Münster, M.: Optimal day-ahead dispatch of an alkaline electrolyser system concerning thermal–electric properties and state-transitional dynamics. Appl. Energy 307, 118091 (2022)
Hong, Z., Wei, Z., Han, X.: Optimization scheduling control strategy of wind-hydrogen system considering hydrogen production efficiency. J. Energy Storage 47(Mar) (2022)
Zhang, C., Wang, J.Y., Ren, Z.B., Yu, Z.Y., Wang, P.J.: Wind-powered 250 kW electrolyzer for dynamic hydrogen production: a pilot study. Int. J. Hydrogen Energy 46(70), 34550–34564 (2021)
Ning, N.: Research on hydrogen generation system by water electrolysis under wide power fluctuation. Ship Sci. Technol. 39(11), 133–136 (2017)
Fang, R.M., Liang, Y.: Control strategy of electrolyzer in a wind-hydrogen system considering the constraints of switching times. Int. J. Hydrogen Energy 44(46), 25104–25111 (2019)
Øystein, U.: Modeling of advanced alkaline electrolyzers: a system simulation approach. Int. J. Hydrogen Energy 28(1), 21–33 (2003)
Jiang, Y., Shen, X.J., Lü, H., Zhang, C.M.: Construction and simulation of operation digital twin model for alkaline water electrolyzer. Trans. China Electrotech. Soc. 37(11), 2897–2908 (2022)
Mónica, S., Ernesto, A., Lourdes, R., Carmen, C.: Semi-empirical model and experimental validation for the performance evaluation of a 15 kW alkaline water electrolyzer. Int. J. Hydrogen Energy 43(45), 20332–20345 (2018)
Hug, W., Bussmann, H., Brinner, A.: Intermittent operation and operation modeling of an alkaline electrolyzer. Int. J. Hydrogen Energy 18(12) (1993)
Huang, D.G., Yin, Y.G., et al.: Effect of lye flow rate on water electrolyzer operation. Henan Chem. Ind. 33(06), 26–28 (2016)
Acknowledgment
This research was supported by the Science and Technology Plan Project of Hebei Province of China (Grant No. 19210108D, 19214501D, 20314501D, F2021202022).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this paper
Cite this paper
Liang, T., Liu, Z., Sun, H., Tan, J. (2024). Multivariate Based Alkaline Electrolyzer Digital Twin Model Construction. In: Sun, H., Pei, W., Dong, Y., Yu, H., You, S. (eds) Proceedings of the 10th Hydrogen Technology Convention, Volume 1. WHTC 2023. Springer Proceedings in Physics, vol 393. Springer, Singapore. https://doi.org/10.1007/978-981-99-8631-6_27
Download citation
DOI: https://doi.org/10.1007/978-981-99-8631-6_27
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8630-9
Online ISBN: 978-981-99-8631-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)