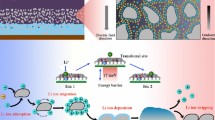
Abstract
With abundant potassium resources and high capacity, potassium metal batteries (PMBs) present a compelling option for the next generation of energy storage technology. However, PMBs suffer from an unstable anode interface caused by uncontrolled dendrite growth, which results in unsatisfactory cyclability and safety concerns. Extensive investigations suggest that significant progress has been made in enhancing the interfacial stability of PMBs. The various effective strategies for stabilizing interfaces can ultimately be attributed to the regulation of the sluggish ion transfer kinetics and irregular deposition, i.e., the arrangement of ion transport behaviors at the interface. Rational modulation of ions transport rate and ions deposition directions makes it possible to obtain a dendrite-free and smooth deposition plane. Herein, the influencing factors and action mechanism of K+ interface transport behaviors are discussed to understand the nature of material design for constructing stable anode interfaces, including regulating the solvation and desolvation structures, accelerating K+ transport kinetics and controlling K+ deposition direction. In addition, the deficiencies and prospects of the research on electrolyte, separators and designed electrode involved in the manufacturing and testing and ion transport process of PMBs are discussed. This review is expected to provide some possible directions for constructing dendrite-free interfaces in advanced PMBs-related research and offer significant insights for prospective experimental research and commercial applications.
Graphical abstract

摘要
凭借丰富的钾资源和高的容量, 钾金属电池(PMBs)成为下一代储能技术的有力候选者。然而, 不受控制的枝晶生长导致PMBs阳极界面不稳定, 从而带来不理想的循环表现和安全问题。广泛的研究表明, 在增**PMBs界面稳定性方面已经取得了重大进展。稳定界面的各种有效策略最终可归因于对缓慢的离子转移动力学和不均匀沉积的调节, 即对离子在界面上输运行为的调控。合理调节离子传输速率和离子沉积方向, 可以获得无枝晶的光滑沉积**面。本文讨论了K+界面输运行为的影响因素和作用机理, 包括调节溶剂化和去溶剂化结构、加速K+传输动力学和控制K+沉积方向, 以了解构筑稳定阳极界面的材料设计本质。此外, 还讨论了PMBs在制造、测试和离子传输过程中涉及到的电极、电解质和隔膜的研究不足和前景。该论文有望为构筑先进的无枝晶PMBs界面提供一些可能的方向, 并为未来的实验研究和商业应用提供重要的见解。


Reproduced with permission from Ref. [48]. Copyright 2010, American Chemical Society. b Schematic representation of heterogeneous nucleation with a spherical crown nucleus. Reproduced with permission from Ref. [53]. Copyright 2019, John Wiley and Sons. c Ionic concentration drop** to zero and cell potential diverges at Sand’s time (about 2100 s). Reproduced with permission from Ref. [59]. Copyright 1999, Elsevier. d A voltage profile of K initial plating; e K dendrite growth mechanism. Reproduced with permission from Ref. [47]. Copyright 2020, Elsevier

Reproduced with permission from Ref. [63]. Copyright 2019, John Wiley and Sons. Schematic diagrams about c various anion structures and d better performance of novel cyclic HFDF− anion-based electrolyte in regulating K+ behaviors than that of conventional ones. Reproduced with permission from Ref. [64]. Copyright 2022, Oxford University Press on behalf of China Science Publishing and Media Ltd. e Schematic diagram of interactions generated between G4 complexes with K+ (left) and their solvation structures in pure G4 or G4 + TTE (right). Reproduced with permission from Ref. [65]. Copyright 2022, American Chemical Society. f Binding energies about FTEP solvents (left) and K+ and corresponding performance of K-Cu cells compared with reported literatures (right). Reproduced with permission from Ref. [95]. Copyright 2022, John Wiley and Sons

Reproduced with permission from Ref. [103]. Copyright 2020, John Wiley and Sons. e Schematic diagrams of preparation process about treated and untreated carbon cloth by NH3 and their f DFT calculation results about K deposition direction. Reproduced with permission from Ref. [33]. Copyright 2019, John Wiley and Sons

Similar content being viewed by others
References
Ryu HH, Sun HH, Myung ST, Yoon CS, Sun YK. Reducing cobalt from lithium-ion batteries for the electric vehicle era. Energy Environ Sci. 2021;14(2):844. https://doi.org/10.1039/D0EE03581E.
Harper G, Sommerville R, Kendrick E, Driscoll L, Slater P, Stolkin R, Walton A, Christensen P, Heidrich O, Lambert S, Abbott A, Ryder K, Gaines L, Anderson P. Recycling lithium-ion batteries from electric vehicles. Nature. 2019;575(7781):75. https://doi.org/10.1038/s41586-019-1682-5.
Lu J, Wu T, Amine K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat Energy. 2017;2(3):17011. https://doi.org/10.1038/nenergy.2017.11.
Pomerantseva E, Bonaccorso F, Feng X, Cui Y, Gogotsi Y. Energy storage: the future enabled by nanomaterials. Science. 2019;366(6468):eaan8285. https://doi.org/10.1126/science.aan8285.
Chen X, Chen XR, Hou TZ, Li BQ, Cheng XB, Zhang R, Zhang Q. Lithiophilicity chemistry of heteroatom-doped carbon to guide uniform lithium nucleation in lithium metal anodes. Sci Adv. 2019;5(2):eaau7728. https://doi.org/10.1126/sciadv.aau7728.
Jiang LL, Yan C, Yao YX, Cai W, Huang JQ, Zhang Q. Inhibiting solvent co-intercalation in a graphite anode by a localized high-concentration electrolyte in fast-charging batteries. Angew Chem Int Ed. 2021;60(7):3402. https://doi.org/10.1002/anie.202009738.
He R, Bai X, Wei AJ, Zhang LH, Liu P, Liu ZF. Y2O3 modification on nickel-rich LiNi0.8Co0.1Mn0.1O2 with improved electrochemical performance in lithium-ion batteries. J Rare Earths. 2022; 40 (2): 309. https://doi.org/10.1016/j.jre.2020.12.010.
Huang Z, Long Y, Zhang M. Recent progress on application and mechanism for potassium storage of carbon-based anode materials. Chin J Rare Met. 2022;46(11):1493. https://doi.org/10.13373/j.cnki.cjrm.XY21040027.
Lin XP, Xue FF, Zhang ZG, Li QH. Sb nanoparticles encapsulated in N-doped carbon nanotubes as freestanding anodes for high-performance lithium and potassium ion batteries. Rare Met. 2023;42(2):449. https://doi.org/10.1007/s12598-022-02143-6.
Sun YQ, Fu W, Hu YX, Vaughan J, Wang LZ. The role of tungsten-related elements for improving the electrochemical performances of cathode materials in lithium ion batteries. Tungsten. 2021; 3(3): 245. https://doi.org/10.1007/s42864-021-00083-9.
Zhang SC, Li SY, Lu YY. Designing safer lithium-based batteries with nonflammable electrolytes: A review. EScience. 2021; 1 (2): 163. https://doi.org/10.1016/j.esci.2021.12.003.
Shi H, Zhang Y, Liu Y, Yuan C. Metallic sodium anodes for advanced sodium metal batteries: progress, challenges and perspective. Chem Rec. 2022;22(10):e202200112. https://doi.org/10.1002/tcr.202200112.
Tang X, Zhang WC, Cao LY. Multifunctional high-fluorine-content molecule with high dipole moment as electrolyte additive for high performance lithium metal batteries. Rare Met. 2022; 41 (7): 726. https://doi.org/10.1007/s12598-021-01843-9.
Min X, **ao J, Fang M, Wang W, Zhao Y, Liu Y, Abdelkader AM, ** K, Kumar RV, Huang Z. Potassium-ion batteries: outlook on present and future technologies. Energy Environ Sci. 2021;14(4):2186. https://doi.org/10.1039/D0EE02917C.
Wang F, Liu Y, Wei HJ, Li TF, **ong XH, Wei SZ, Ren FZ, Volinsky AA. Recent advances and perspective in metal coordination materials-based electrode materials for potassium-ion batteries. Rare Met. 2021;40(2):448. https://doi.org/10.1007/s12598-020-01649-1.
Liu X, Tong Y, Wu Y, Zheng J, Sun Y, Niu L, Li H. Synergistically enhanced electrochemical performance using nitrogen, phosphorus and sulfur tri-doped hollow carbon for advanced potassium ion storage device. Chem Eng J. 2022;431:133986. https://doi.org/10.1016/j.cej.2021.133986.
Sun Y, Zheng J, Tong Y, Wu Y, Liu X, Niu L, Li H. Construction of three-dimensional nitrogen doped porous carbon flake electrodes for advanced potassium-ion hybrid capacitors. J Colloid Interface Sci. 2022;606:1940. https://doi.org/10.1016/j.jcis.2021.09.143.
Nathan MGT, Yu H, Kim GT, Kim JH, Cho JS, Kim J, Kim JK. Recent advances in layered metal-oxide cathodes for application in potassium-ion batteries. Adv Sci. 2022;9(18):2105882. https://doi.org/10.1002/advs.202105882.
Zhang LS, Gao XL, Liu XH, Zhang ZJ, Cao R. Cheng HC, Wang MY, Yan XY, Yang SC. CHAIN: unlocking informatics-aided design of Li metal anode from materials to applications. Rare Met. 2022; 41(5): 1477. https://doi.org/10.1007/s12598-021-01925-8.
Chen M, Wang E, Liu Q, Guo X, Chen W, Chou SL, Dou SX. Recent progress on iron- and manganese-based anodes for sodium-ion and potassium-ion batteries. Energy Storage Mater. 2019;19:163. https://doi.org/10.1016/j.ensm.2019.03.030.
Zheng J, Sun Y, Wu Y, Rong J, Wang Z, Li H, Niu L. Ultralong cycle life and high rate potassium ion batteries enabled by multi-level porous carbon. J Power Sources. 2021;492:229614. https://doi.org/10.1016/j.jpowsour.2021.229614.
Lv JR, Wang B, Hao JX, Ding HB, Fan L, Tao RQ, Yang HG, Zhou J, Lu BA. Single-crystalline Mn-based oxide as a high-rate and long-life cathode material for potassium-ion battery. EScience. 2023; 3 (1): 100081. https://doi.org/10.1016/j.esci.2022.10.007.
Yu A, Pan Q, Zhang M, **e D, Tang Y. Fast rate and long life potassium-ion based dual-ion battery through 3D porous organic negative electrode. Adv Funct Mater. 2020;30(24):2001440. https://doi.org/10.1002/adfm.202001440.
Liu P, Mitlin D. Emerging potassium metal anodes: perspectives on control of the electrochemical interfaces. Acc Chem Res. 2020;53(6):1161. https://doi.org/10.1021/acs.accounts.0c00099.
Liu X, Yu X, Tong Y, Sun Y, Mai W, Niu L, Li H. Potassium storage in bismuth nanoparticles embedded in N-doped porous carbon facilitated by ether-based electrolyte. Chem Eng J. 2022;446:137329. https://doi.org/10.1016/j.cej.2022.137329.
Wang D, Tian KH, Wang J, Wang ZY, Luo SH, Liu YG, Wang Q, Zhang YH, Hao AM, Yi TF. Sulfur-doped 3D hierarchical porous carbon network toward excellent potassium-ion storage performance. Rare Met. 2021; 40 (9): 2464. https://doi.org/10.1007/s12598-021-01715-2.
Liu W, Liu P, Mitlin D. Tutorial review on structure—dendrite growth relations in metal battery anode supports. Chem Soc Rev. 2020;49(20):7284. https://doi.org/10.1039/D0CS00867B.
Liu W, Liu P, Mitlin D. Review of emerging concepts in sei analysis and artificial sei membranes for lithium, sodium, and potassium metal battery anodes. Adv Energy Mater. 2020;10(43):2002297. https://doi.org/10.1002/aenm.202002297.
Wei C, Tao Y, An Y, Tian Y, Zhang Y, Feng J, Qian Y. Recent advances of emerging 2D mxene for stable and dendrite-free metal anodes. Adv Funct Mater. 2020;30(45):2004613. https://doi.org/10.1002/adfm.202004613.
Han Y, Liu B, **ao Z, Zhang W, Wang X, Pan G, **a Y, **a X, Tu J. Interface issues of lithium metal anode for high-energy batteries: challenges, strategies, and perspectives. InfoMat. 2021;3(2):155. https://doi.org/10.1002/inf2.12166.
Zhou W, Li Y, **n S, Goodenough JB. Rechargeable sodium all-solid-state battery. ACS Cent Sci. 2017;3(1):52. https://doi.org/10.1021/acscentsci.6b00321.
Wei C, Tao Y, Fei H, An Y, Tian Y, Feng J, Qian Y. Recent advances and perspectives in stable and dendrite-free potassium metal anodes. Energy Storage Mater. 2020;30:206. https://doi.org/10.1016/j.ensm.2020.05.018.
Wang H, Hu J, Dong J, Lau KC, Qin L, Lei Y, Li B, Zhai D, Wu Y, Kang F. Artificial solid-electrolyte interphase enabled high-capacity and stable cycling potassium metal batteries. Adv Energy Mater. 2019;9(43):1902697. https://doi.org/10.1002/aenm.201902697.
**ao N, Zheng J, Gourdin G, Schkeryantz L, Wu Y. Anchoring an artificial protective layer to stabilize potassium metal anode in rechargeable K-O2 batteries. ACS Appl Mater Interfaces. 2019;11(18):16571. https://doi.org/10.1021/acsami.9b02116.
Li S, Zhu H, Liu Y, Han Z, Peng L, Li S, Yu C, Cheng S, **e J. Codoped porous carbon nanofibres as a potassium metal host for nonaqueous K-ion batteries. Nat Commun. 2022;13(1):4911. https://doi.org/10.1038/s41467-022-32660-y.
Li L, Zhao S, Hu Z, Chou S-L, Chen J. Develo** better ester- and ether-based electrolytes for potassium-ion batteries. Chem Sci. 2021;12(7):2345. https://doi.org/10.1039/D0SC06537D.
Zhang Y, Bahi A, Ko F, Liu J. Polyacrylonitrile-reinforced composite gel polymer electrolytes for stable potassium metal anodes. Small. 2022;18(8):2107186. https://doi.org/10.1002/smll.202107186.
Li J, Hu Y, **e H, Peng J, Fan L, Zhou J, Lu B. Weak cation–solvent interactions in ether-based electrolytes stabilizing potassium-ion batteries. Angew Chem Int Ed. 2022;61(33):e202208291. https://doi.org/10.1002/anie.202208291.
Gu Y, Wang WW, Li YJ, Wu QH, Tang S, Yan JW, Zheng MS, Wu DY, Fan CH, Hu WQ, Chen ZB, Fang Y, Zhang QH, Dong QF, Mao BW. Designable ultra-smooth ultra-thin solid-electrolyte interphases of three alkali metal anodes. Nat Commun. 2018;9(1):1339. https://doi.org/10.1038/s41467-018-03466-8.
Shi P, Zhang S, Lu G, Wang L, Jiang Y, Liu F, Yao Y, Yang H, Ma M, Ye S, Tao X, Feng Y, Wu X, Rui X, Yu Y. Red phosphorous-derived protective layers with high ionic conductivity and mechanical strength on dendrite-free sodium and potassium metal anodes. Adv Energy Mater. 2021;11(5):2003381. https://doi.org/10.1002/aenm.202003381.
Yang Y, Huang C, Zhang Y, Wu Y, Zhao X, Qian Y, Chang G, Tang Q, Hu A, Chen X. Processable potassium–carbon nanotube film with a three-dimensional structure for ultrastable metallic potassium anodes. ACS Appl Mater Interfaces. 2022;14(50):55577. https://doi.org/10.1021/acsami.2c16255.
Qin L, Lei Y, Wang H, Dong J, Wu Y, Zhai D, Kang F, Tao Y, Yang QH. Capillary encapsulation of metallic potassium in aligned carbon nanotubes for use as stable potassium metal anodes. Adv Energy Mater. 2019;9(29):1901427. https://doi.org/10.1002/aenm.201901427.
Wang J, Chen M, Lu Z, Chen Z, Si L. Robust potassium metal anodes realized by ferroelectricity and high conductivity separator. Mater Sci Semicond Proc. 2022;151:107001. https://doi.org/10.1016/j.mssp.2022.107001.
Luo Y, Mou P, Yuan W, Li L, Fan Y, Chen Y, Chen X, Shu J, Zhang L. Anti-liquid metal permeation separator for stretchable potassium metal batteries. Chem Eng J. 2023;452:139157. https://doi.org/10.1016/j.cej.2022.139157.
Guan X, Wang A, Liu S, Li G, Liang F, Yang YW, Liu X, Luo J. Controlling nucleation in lithium metal anodes. Small. 2018;14(37):1801423. https://doi.org/10.1002/smll.201801423.
Wang C, Wang A, Ren L, Guan X, Wang D, Dong A, Zhang C, Li G, Luo J. Controlling Li ion flux through materials innovation for dendrite-free lithium metal anodes. Adv Funct Mater. 2019;29(49):1905940. https://doi.org/10.1002/adfm.201905940.
Hu J, Wang H, Wang S, Lei Y, Qin L, Li X, Zhai D, Li B, Kang F. Electrochemical deposition mechanism of sodium and potassium. Energy Storage Mater. 2021;36:91. https://doi.org/10.1016/j.ensm.2020.12.017.
Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem Mater. 2010;22(3):587. https://doi.org/10.1021/cm901452z.
Peled E, Golodnitsky D, Ardel G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J Electrochem Soc. 1997;144(8):L208. https://doi.org/10.1149/1.1837858.
Yu X, Manthiram A. Electrode–electrolyte interfaces in lithium–sulfur batteries with liquid or inorganic solid electrolytes. Acc Chem Res. 2017;50(11):2653. https://doi.org/10.1021/acs.accounts.7b00460.
Wang H, Matios E, Luo J, Li W. Combining theories and experiments to understand the sodium nucleation behavior towards safe sodium metal batteries. Chem Soc Rev. 2020;49(12):3783. https://doi.org/10.1039/D0CS00033G.
Scharifker B, Hills G. Theoretical and experimental studies of multiple nucleation. Electrochim Acta. 1983;28(7):879. https://doi.org/10.1016/0013-4686(83)85163-9.
Sun X, Zhang X, Ma Q, Guan X, Wang W, Luo J. Revisiting the electroplating process for lithium-metal anodes for lithium-metal batteries. Angew Chem Int Ed. 2020;59(17):6665. https://doi.org/10.1002/anie.201912217.
Zhong Y, Zhou S, He Q, Pan A. Architecture design principles for stable electrodeposition behavior-towards better alkali metal (Li/Na/K) anodes. Energy Storage Mater. 2022;45:48. https://doi.org/10.1016/j.ensm.2021.11.033.
Wang J, Yang Y, Zhang Y, Li Y, Sun R, Wang Z, Wang H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Storage Mater. 2021;35:19. https://doi.org/10.1016/j.ensm.2020.10.027.
Chazalviel JN. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys Rev A. 1990;42(12):7355. https://doi.org/10.1103/PhysRevA.42.7355.
Li G. Regulating mass transport behavior for high-performance lithium metal batteries and fast-charging lithium-ion batteries. Adv Energy Mater. 2021;11(7):2002891. https://doi.org/10.1002/aenm.202002891.
**ao J. How lithium dendrites form in liquid batteries. Science. 2019;366(6464):426. https://doi.org/10.1126/science.aay8672.
Brissot C, Rosso M, Chazalviel JN, Lascaud S. Dendritic growth mechanisms in lithium/polymer cells. J Power Sources. 1999;81–82:925. https://doi.org/10.1016/S0378-7753(98)00242-0.
Wang J, Li L, Hu H, Hu H, Guan Q, Huang M, Jia L, Adenusi H, Tian KV, Zhang J, Passerini S, Lin H. Toward dendrite-free metallic lithium anodes: from structural design to optimal electrochemical diffusion kinetics. ACS Nano. 2022;16(11):17729. https://doi.org/10.1021/acsnano.2c08480.
Wang J, Zhang J, Duan S, Jia L, **ao Q, Liu H, Hu H, Cheng S, Zhang Z, Li L, Duan W, Zhang Y, Lin H. Lithium atom surface diffusion and delocalized deposition propelled by atomic metal catalyst toward ultrahigh-capacity dendrite-free lithium anode. Nano Lett. 2022;22(19):8008. https://doi.org/10.1021/acs.nanolett.2c02611.
Ma B, Sittisomwong P, Ma J, Bai P. Effects of interfacial solvation structures on the morphological stability of potassium metal anodes revealed by operando diagnosis. ACS Appl Energy Mater. 2022;5(6):7124. https://doi.org/10.1021/acsaem.2c00716.
Wang H, Yu D, Wang X, Niu Z, Chen M, Cheng L, Zhou W, Guo L. Electrolyte chemistry enables simultaneous stabilization of potassium metal and alloying anode for potassium-ion batteries. Angew Chem Int Ed. 2019;58(46):16451. https://doi.org/10.1002/anie.201908607.
Hu Y, Fan L, Rao AM, Yu W, Zhuoma C, Feng Y, Qin Z, Zhou J, Lu B. Cyclic-anion salt for high-voltage stable potassium-metal batteries. Natl Sci Rev. 2022;9(10):nwac134. https://doi.org/10.1093/nsr/nwac134.
Chen C, Zhou J, Gong W, Fan X, Meng X, Chen S, Sun L, Meng Y, Tao K, Ülgüt B, Sun P, Bielawski CW, Geng J. Regulating the solvation structure of potassium ions using a multidentate ether in potassium metal batteries. ACS Appl Energy Mater. 2022;5(9):10366. https://doi.org/10.1021/acsaem.2c01504.
Fan L, **e H, Hu Y, Caixiang Z, Rao AM, Zhou J, Lu B. A tailored electrolyte for safe and durable potassium ion batteries. Energy Environ Sci. 2023;16(1):305. https://doi.org/10.1039/D2EE03294E.
Yu D, Wang H, Zhang W, Dong H, Zhu Q, Yang J, Huang S. Unraveling the role of ion-solvent chemistry in stabilizing small-molecule organic cathode for potassium-ion batteries. Energy Storage Mater. 2021;43:172. https://doi.org/10.1016/j.ensm.2021.08.040.
Xu W, Wang H, Hu J, Zhang H, Zhang B, Kang F, Zhai D. A highly concentrated electrolyte for high-efficiency potassium metal batteries. Chem Commun. 2021;57(8):1034. https://doi.org/10.1039/D0CC07266D.
Yamada Y, Furukawa K, Sodeyama K, Kikuchi K, Yaegashi M, Tateyama Y, Yamada A. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J Am Chem Soc. 2014;136(13):5039. https://doi.org/10.1021/ja412807w.
Bao C, Wang B, Liu P, Wu H, Zhou Y, Wang D, Liu H, Dou S. Solid electrolyte interphases on sodium metal anodes. Adv Funct Mater. 2020;30(52):2004891. https://doi.org/10.1002/adfm.202004891.
Gao Y, Hou Z, Zhou R, Wang D, Guo X, Zhu Y, Zhang B. Critical roles of mechanical properties of solid electrolyte interphase for potassium metal anodes. Adv Funct Mater. 2022;32(17):2112399. https://doi.org/10.1002/adfm.202112399.
Gao Y, Du X, Hou Z, Shen X, Mai YW, Tarascon JM, Zhang B. Unraveling the mechanical origin of stable solid electrolyte interphase. Joule. 2021;5(7):1860. https://doi.org/10.1016/j.joule.2021.05.015.
Wang H, Dong J, Guo Q, Xu W, Zhang H, Lau KC, Wei Y, Hu J, Zhai D, Kang F. Highly stable potassium metal batteries enabled by regulating surface chemistry in ether electrolyte. Energy Storage Mater. 2021;42:526. https://doi.org/10.1016/j.ensm.2021.08.013.
Yoshii K, Masese T, Kato M, Kubota K, Senoh H, Shikano M. Sulfonylamide-based ionic liquids for high-voltage potassium-ion batteries with honeycomb layered cathode oxides. ChemElectroChem. 2019;6(15):3901. https://doi.org/10.1002/celc.201900689.
Sun H, Liang P, Zhu G, Hung WH, Li YY, Tai HC, Huang CL, Li J, Meng Y, Angell M, Wang CA, Dai H. A high-performance potassium metal battery using safe ionic liquid electrolyte. Proc Natl Acad Sci USA. 2020;117(45):27847. https://doi.org/10.1073/pnas.2012716117.
Yang Q, Ding Y, He G. An amalgam route to stabilize potassium metal anodes over a wide temperature range. Chem Commun. 2020;56(24):3512. https://doi.org/10.1039/D0CC00171F.
Yang H, He F, Li M, Huang F, Chen Z, Shi P, Liu F, Jiang Y, He L, Gu M, Yu Y. Design principles of sodium/potassium protection layer for high-power high-energy sodium/potassium-metal batteries in carbonate electrolytes: a case study of Na2Te/K2Te. Adv Mater. 2021;33(48):2106353. https://doi.org/10.1002/adma.202106353.
Jiang Y, Yang Y, Ling F, Lu G, Huang F, Tao X, Wu S, Cheng X, Liu F, Li D, Yang H, Yao Y, Shi P, Chen Q, Rui X, Yu Y. Artificial heterogeneous interphase layer with boosted ion affinity and diffusion for Na/K-metal batteries. Adv Mater. 2022;34(13):2109439. https://doi.org/10.1002/adma.202109439.
Li D, Sun Y, Li M, Cheng X, Yao Y, Huang F, Jiao S, Gu M, Rui X, Ali Z, Ma C, Wu ZS, Yu Y. Rational design of an artificial sei: alloy/solid electrolyte hybrid layer for a highly reversible Na and K metal anode. ACS Nano. 2022;16(10):16966. https://doi.org/10.1021/acsnano.2c07049.
**ao N, Gourdin G, Wu Y. Simultaneous stabilization of potassium metal and superoxide in K–O2 batteries on the basis of electrolyte reactivity. Angew Chem Int Ed. 2018;57(34):10864. https://doi.org/10.1002/anie.201804115.
Popovic J. Review—recent advances in understanding potassium metal anodes. J Electrochem Soc. 2022;169(3):030510. https://doi.org/10.1149/1945-7111/ac580f.
Park J, Jeong Y, Alfaruqi MH, Liu Y, Xu X, **ong S, Jung MG, Jung HG, Kim J, Hwang JY, Sun YK. Stable solid electrolyte interphase for long-life potassium metal batteries. ACS Energy Lett. 2022;7(1):401. https://doi.org/10.1021/acsenergylett.1c02354.
Qin C, Wang D, Liu Y, Yang P, **e T, Huang L, Zou H, Li G, Wu Y. Tribo-electrochemistry induced artificial solid electrolyte interface by self-catalysis. Nat Commun. 2021;12(1):7184. https://doi.org/10.1038/s41467-021-27494-z.
Gu M, Rao AM, Zhou J, Lu B. In situ formed uniform and elastic SEI for high-performance batteries. Energy Environ Sci. 2023;16(3):1166. https://doi.org/10.1039/D2EE04148K.
Ding H, Wang J, Zhou J, Wang C, Lu B. Building electrode skins for ultra-stable potassium metal batteries. Nat Commun. 2023;14(1):2305. https://doi.org/10.1038/s41467-023-38065-9.
Liu P, Hao H, Celio H, Cui J, Ren M, Wang Y, Dong H, Chowdhury AR, Hutter T, Perras FA, Nanda J, Watt J, Mitlin D. Multifunctional separator allows stable cycling of potassium metal anodes and of potassium metal batteries. Adv Mater. 2022;34(7):2105855. https://doi.org/10.1002/adma.202105855.
Ding Y, Guo X, Qian Y, Xue L, Dolocan A, Yu G. Room-temperature all-liquid-metal batteries based on fusible alloys with regulated interfacial chemistry and wetting. Adv Mater. 2020;32(30):2002577. https://doi.org/10.1002/adma.202002577.
Park J, Lee J, Alfaruqi MH, Kwak WJ, Kim J, Hwang JY. Initial investigation and evaluation of potassium metal as an anode for rechargeable potassium batteries. J Mater Chem A. 2020;8(33):16718. https://doi.org/10.1039/D0TA03562A.
Xue L, Gao H, Zhou W, **n S, Park K, Li Y, Goodenough JB. Liquid K-Na alloy anode enables dendrite-free potassium batteries. Adv Mater. 2016;28(43):9608. https://doi.org/10.1002/adma.201602633.
Qin L, Yang W, Lv W, Liu L, Lei Y, Yu W, Kang F, Kim JK, Zhai D, Yang QH. Room-temperature liquid metal-based anodes for high-energy potassium-based electrochemical devices. Chem Commun. 2018;54(58):8032. https://doi.org/10.1039/C8CC03545H.
Zhang L, Li Y, Zhang S, Wang X, **a X, **e D, Gu C, Tu J. Non-newtonian fluid state K–Na alloy for a stretchable energy storage device. Small Methods. 2019;3(10):1900383. https://doi.org/10.1002/smtd.201900383.
Zhang L, **a X, Zhong Y, **e D, Liu S, Wang X, Tu J. Exploring self-healing liquid Na–K alloy for dendrite-free electrochemical energy storage. Adv Mater. 2018;30(46):1804011. https://doi.org/10.1002/adma.201804011.
Zhang L, Peng S, Ding Y, Guo X, Qian Y, Celio H, He G, Yu G. A graphite intercalation compound associated with liquid Na–K towards ultra-stable and high-capacity alkali metal anodes. Energy Environ Sci. 2019;12(6):1989. https://doi.org/10.1039/C9EE00437H.
Luo T, Zhao Q, Liu Y, Meng W, Sun Q, Dai L, Liu S, Wang L. High-rate and dendrite-free liquid alloy anode for high energy potassium metal batteries. EcoMat. 2022;4(5):e12203. https://doi.org/10.1002/eom2.12203.
Yuan W, Ding T, Mou P, Luo Y, Li L, Chen Y, Chen X, Shu J, Zhang L. Semi-solid CNT@NaK anode for potassium metal battery. Adv Funct Mater. 2023;33(4):2209774. https://doi.org/10.1002/adfm.202209774.
Qian S, Chen H, Zheng M, Zhu Y, **ng C, Tian Y, Yang P, Wu Z, Zhang S. Complementary combination of lithium protection strategies for robust and longevous lithium metal batteries. Energy Storage Mater. 2023;57:229. https://doi.org/10.1016/j.ensm.2023.02.019.
Wang J, Yuan J, Chen C, Wang L, Zhai Z, Fu Q, Liu Y, Dong L, Yan W, Li A, Zhang J. Cu3Pt alloy-functionalized Cu mesh as current collector for dendritic-free anodes of potassium metal batteries. Nano Energy. 2020;75:104914. https://doi.org/10.1016/j.nanoen.2020.104914.
Li H, Liu Y, Wang J, Yan W, Zhang J. Robust 3D copper foam functionalized with gold nanoparticles as anode for high-performance potassium metal batteries. Chem Asian J. 2022;17(15):e202200430. https://doi.org/10.1002/asia.202200430.
Wang J, Yan W, Zhang J. High area capacity and dendrite-free anode constructed by highly potassiophilic Pd/Cu current collector for low-temperature potassium metal battery. Nano Energy. 2022;96:107131. https://doi.org/10.1016/j.nanoen.2022.107131.
Liu P, Wang Y, Gu Q, Nanda J, Watt J, Mitlin D. Dendrite-free potassium metal anodes in a carbonate electrolyte. Adv Mater. 2020;32(7):1906735. https://doi.org/10.1002/adma.201906735.
Yi Y, Li J, Gao Z, Liu W, Zhao Y, Wang M, Zhao W, Han Y, Sun J, Zhang J. Highly potassiophilic graphdiyne skeletons decorated with Cu quantum dots enable dendrite-free potassium-metal anodes. Adv Mater. 2022;34(29):2202685. https://doi.org/10.1002/adma.202202685.
Wang S, Yan Y, **ong D, Li G, Wang Y, Chen F, Chen S, Tian B, Shi Y. Towards dendrite-free potassium-metal batteries: rational design of a multifunctional 3D polyvinyl alcohol-borax layer. Angew Chem Int Ed. 2021;60(47):25122. https://doi.org/10.1002/anie.202111753.
Liu P, Wang Y, Hao H, Basu S, Feng X, Xu Y, Boscoboinik JA, Nanda J, Watt J, Mitlin D. Stable potassium metal anodes with an all-aluminum current collector through improved electrolyte wetting. Adv Mater. 2020;32(49):2002908. https://doi.org/10.1002/adma.202002908.
Zhou R, Tan H, Gao Y, Hou Z, Du X, Zhang B. Constructing resilient solid electrolyte interphases on carbon nanofiber film for advanced potassium metal anodes. Carbon. 2022;186:141. https://doi.org/10.1016/j.carbon.2021.10.023.
Tang X, Zhou D, Li P, Guo X, Sun B, Liu H, Yan K, Gogotsi Y, Wang G. Mxene-based dendrite-free potassium metal batteries. Adv Mater. 2020;32(4):1906739. https://doi.org/10.1002/adma.201906739.
Shi H, Dong Y, Zheng S, Dong C, Wu ZS. Three dimensional Ti3C2 mxene nanoribbon frameworks with uniform potassiophilic sites for the dendrite-free potassium metal anodes. Nanoscale Adv. 2020;2(9):4212. https://doi.org/10.1039/D0NA00515K.
Qiao F, Meng J, Wang J, Wu P, Xu D, An Q, Wang X, Mai L. Building carbon cloth-based dendrite-free potassium metal anodes for potassium metal pouch cells. J Mater Chem A. 2021;9(40):23046. https://doi.org/10.1039/D1TA06327H.
Zhao X, Chen F, Liu J, Cheng M, Su H, Liu J, Xu Y. Enhanced surface binding energy regulates uniform potassium deposition for stable potassium metal anodes. J Mater Chem A. 2020;8(11):5671. https://doi.org/10.1039/C9TA14226F.
Li Y, Zhang L, Liu S, Wang X, **e D, **a X, Gu C, Tu J. Original growth mechanism for ultra-stable dendrite-free potassium metal electrode. Nano Energy. 2019;62:367. https://doi.org/10.1016/j.nanoen.2019.05.020.
Zhang J, Li Y, Zhu L, Wang X, Tu J. Potassiophilic skeleton achieving highly stable potassium metal anode. Chem Eng J. 2022;449:137659. https://doi.org/10.1016/j.cej.2022.137659.
Wang L, Wang H, Cheng M, Hong Y, Li M, Su H, Sun J, Wang J, Xu Y. Metal–organic framework@polyacrylonitrile-derived potassiophilic nanoporous carbon nanofiber paper enables stable potassium metal anodes. ACS Appl Energy Mater. 2021;4(6):6245. https://doi.org/10.1021/acsaem.1c01002.
Zhou M, Qi W, Hu Z, Cheng M, Zhao X, **ong P, Su H, Li M, Hu J, Xu Y. Highly potassiophilic carbon nanofiber paper derived from bacterial cellulose enables ultra-stable dendrite-free potassium metal anodes. ACS Appl Mater Interfaces. 2021;13(15):17629. https://doi.org/10.1021/acsami.1c02186.
Meng J, Zhu H, **ao Z, Zhang X, Niu C, Liu Y, Jiang G, Wang X, Qiao F, Hong X, Liu F, Pang Q, Mai L. Amine-wetting-enabled dendrite-free potassium metal anode. ACS Nano. 2022;16(5):7291. https://doi.org/10.1021/acsnano.2c01432.
**ong J, Ye M, Wang Z, Chen J, Zhang Y, Tang Y, Li CC. Fast and homogeneous ion regulation toward a 4V, high-rate and dendrite-free potassium metal battery. Chem Eng J. 2022;442:135927. https://doi.org/10.1016/j.cej.2022.135927.
Chen X, Bai YK, Shen X, Peng HJ, Zhang Q. Sodiophilicity/potassiophilicity chemistry in sodium/potassium metal anodes. J Energy Chem. 2020;51:1. https://doi.org/10.1016/j.jechem.2020.03.051.
Zhang D, Ma X, Wu L, Wen J, Li F, Zhou J, Rao AM, Lu B. Coupling low-tortuosity carbon matrix with single-atom chemistry enables dendrite-free potassium-metal anode. Adv Energy Mater. 2023;13(2):2203277. https://doi.org/10.1002/aenm.202203277.
Han M, Jiang J, Lu S, Jiang Y, Ma W, Liu X, Zhao B, Zhang J. Moderate specific surface areas help three-dimensional frameworks achieve dendrite-free potassium-metal anodes. ACS Appl Mater Interfaces. 2022;14(1):900. https://doi.org/10.1021/acsami.1c19742.
Ye M, Hwang JY, Sun YK. A 4 V class potassium metal battery with extremely low overpotential. ACS Nano. 2019;13(8):9306. https://doi.org/10.1021/acsnano.9b03915.
**e J, Ji Y, Ma L, Wen Z, Pu J, Wang L, Ding S, Shen Z, Liu Y, Li J, Mai W, Hong G. Bifunctional alloy/solid-electrolyte interphase layer for enhanced potassium metal batteries via prepassivation. ACS Nano. 2023;17(2):1511. https://doi.org/10.1021/acsnano.2c10535.
Wei Z, Wang A, Guan X, Li G, Yang Z, Huang C, Zhang J, Ren L, Luo J, Liu X. Processable potassium metal anode for stable batteries. Energy Environ Mater. 2022;5(4):1278. https://doi.org/10.1002/eem2.12244.
Zhao Y, Liu B, Yi Y, Lian X, Wang M, Li S, Yang X, Sun J. An anode-free potassium-metal battery enabled by a directly grown graphene-modulated aluminum current collector. Adv Mater. 2022;34(29):2202902. https://doi.org/10.1002/adma.202202902.
Gireaud L, Grugeon S, Laruelle S, Yrieix B, Tarascon JM. Lithium metal strip**/plating mechanisms studies: a metallurgical approach. Electrochem Commun. 2006;8(10):1639. https://doi.org/10.1016/j.elecom.2006.07.037.
Liu P, Yen D, Vishnugopi BS, Kankanallu VR, Gursoy D, Ge M, Watt J, Mukherjee PP, Chen-Wiegart YK, Mitlin D. Influence of potassium metal-support interactions on dendrite growth. Angew Chem Int Ed. 2023;135:e202300943. https://doi.org/10.1002/anie.202300943.
Yuan H, Li H, Zhang T, Li G, He T, Du F, Feng S. A K2Fe4O7 superionic conductor for all-solid-state potassium metal batteries. J Mater Chem A. 2018;6(18):8413. https://doi.org/10.1039/C8TA01418C.
Hamada M, Tatara R, Kubota K, Kumakura S, Komaba S. All-solid-state potassium polymer batteries enabled by the effective pretreatment of potassium metal. ACS Energy Lett. 2022;7(7):2244. https://doi.org/10.1021/acsenergylett.2c01096.
Khudyshkina AD, Morozova PA, Butzelaar AJ, Hoffmann M, Wilhelm M, Theato P, Fedotov SS, Jeschull F. Poly(ethylene oxide)-based electrolytes for solid-state potassium metal batteries with a prussian blue positive electrode. ACS Appl Polym Mater. 2022;4(4):2734. https://doi.org/10.1021/acsapm.2c00014.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 52272194), Liaoning Revitalization Talents Program (No. XLYC2007155) and the Fundamental Research Funds for the Central Universities (Nos. N2025018 and N2025009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, LK., Gao, XW., Ren, TZ. et al. Regulating ion transport behaviors toward dendrite-free potassium metal batteries: recent advances and perspectives. Rare Met. 43, 1435–1460 (2024). https://doi.org/10.1007/s12598-023-02537-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02537-0