Abstract
Nitro-fatty acids (NO2-FAs) are a class of bioactive lipids that mediate metabolic, anti-oxidative stress, anti-inflammatory, and other signaling actions. Endogenously, NO2-FAs are derived from the non-enzymatic reactions of unsaturated fatty acids with reactive nitrogen species. The electrophilic properties of the nitro group results in NO2-FAs being able to undergo rapid and reversible reactions with biological nucleophiles, such as cysteine and histidine, thus supporting post-translational modifications of proteins. The reactions of NO2-FAs with biological nucleophiles regulate a range of key signaling pathways involved in gene expression responses, enzyme activity, and cellular processes. In disease animal models, NO2-FAs are produced under conditions of inflammation and oxidative stress and play a protective role in a variety of metabolic diseases, which have been associated with anti-atherosclerosis, blood-pressure lowering, and are involved in the regulation of glycolipid metabolism and insulin resistance. Based on these, more clinical studies might find a correlation between NO2-FAs levels and pathophysiology in patients with metabolic diseases. Importantly, NO2-FAs therapeutics are effective in clinical trials. In addition, dietary supplementation with nitrates and unsaturated fatty acids can endogenously increase NO2-FAs levels in mice and humans. These findings support dietary approaches that increase the endogenous levels of NO2-FAs might potentially reduce the risk of metabolic diseases. To identify the specific mechanism of action and therapeutic potential of NO2-FAs, we have summarized the main mechanisms of action of NO2-FAs in metabolic disease progression to provide insights for the development of new therapeutics for metabolic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metabolic diseases, such as cardiovascular diseases (CVDs), non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM) and obesity, pose a great threat to human health globally (Chew et al. 2023). The worldwide prevalence of metabolic diseases has risen over the past two decades (Chew et al. 2023), and it is important to find drugs that can effectively treat metabolic diseases (Zeng et al. 2023). In recent years, drugs such as sulphonylureas, statins, and biguanides have been commonly used to treat metabolic diseases (Grundy et al. 2019; McGowan & Roumie 2018; Palmer & Strippoli 2018). However, some adverse effects may occur with these drugs, such as gastrointestinal disorders, hypoglycemia, and liver dysfunction because of the complex nature of human drug metabolism, diet-drug interactions, and disease pathology (Adhyaru & Jacobson 2018; Khunti et al. 2018; Wang & Hoyte 2019). Therefore, there is an urgent need to develop safe and effective drugs for the treatment of metabolic diseases. The dietary intake of specific polyunsaturated fatty acids (PUFAs) can have a protective effect on a wide range of diseases, including heart disease and metabolic disorders (Bhatt et al. 2019; Poudyal & Brown 2015). Moreover, a moderate intake of PUFAs has few side effects (Weinberg et al. 2021). The 2021 European Society of Cardiology guidelines for the prevention of CVDs also recommend an intake of dietary PUFAs to replace saturated fatty acids (Poudyal & Brown 2015; Visseren et al. 2021). The therapeutic potential of PUFAs is primarily derived from the metabolic derivatives of PUFAs and lipid mediator bioactive compounds (Halade et al. 2018).
Nitro-fatty acids (NO2-FAs), which are produced from the reactions of PUFAs with reactive nitrogen species, are a class of PUFA derivatives that are endogenously generated during digestion, inflammation, and oxidative stress (Fraga et al. 2023; Piesche et al. 2020). NO2-FAs play a protective role in metabolic diseases, such as CVDs (Nettersheim et al. 2022), NAFLD (Rom et al. 2019), and T2DM (Schopfer et al. 2010), and have attracted extensive attention from the scientific community in the past decade. A variety of NO2-FAs have been detected in humans, animals, and plants. Higher levels of nitro-linoleic acid (NO2-LA) were observed in the hyperlipidemia group compared to the normolipidemic counterparts (Lima et al. 2002). Free NO2-LA and nitro-oleic acid (NO2-OA) levels were all negatively associated with baseline disease activity measured in 28 joints in patients with rheumatoid arthritis (Fu et al. 2016). However, the correlation between NO2-FAs levels and metabolic diseases in humans is not clear, and more clinical trial results are needed to elucidate. In disease animal models, NO2-FAs are involved in multiple protective mechanisms related to anti-atherosclerosis (Rudolph et al. 2010a, b), blood-pressure lowering (Kansanen et al. 2017), anti-inflammatory (Villacorta et al. 2018), and anti-insulin resistance (Khoo et al. 2019) effects, and are involved in the regulation of glycolipid metabolism (Arbeeny et al. 2019). The electrophilic properties of NO2-FAs enable NO2-FAs to undergo reversible Michael addition reactions with cysteine and histidine residues, resulting in the post-translational modification (PTM) of proteins (Koutoulogenis & Kokotos 2021). The NO2-FA-induced PTM of signaling proteins can lead to modifications in the protein structure and function, triggering a cascade of downstream signaling events, including gene expression, enzyme activity, and regulation of cellular processes (Grippo et al. 2021). Specifically, the main mechanisms of cell signal transduction involving NO2-FAs include inhibiting and regulating inflammatory nuclear factor kappa-B (NF-κB), signal transducer and activator of transcription (STAT), reducing coenzyme 2 (NADPH) and soluble epoxide hydrolase (sEH), activating peroxisome proliferation-activated receptor gamma (PPAR-γ) and the Kelch-like ECH-associated protein-1 (Keap1)-nuclear factor-erythroid 2-related factor 2 (Nrf2) antioxidant pathway (Delmastro-Greenwood et al. 2014). NO2-FAs have been shown to mediate signaling actions in preclinical models of inflammatory, oxidative stress-related, and metabolic diseases, and have shown potential as therapeutic agents. 10-nitro-9(E)-octadec-9-enoic acid (CXA-10), a specific regioisomer of NO2-OA has been developed clinically as a drug for the treatment of inflammation (Garner et al. 2019). In healthy and obese subjects, CXA-10 was found to be safe and well tolerated, and reduced the levels of inflammation-related biomarkers in obese subjects (Garner et al. 2019). Recently, the use of emerging technologies has enabled the delivery of NO2-FAs nanoparticles for improving vascular function after ischemia-reperfusion injury (Yu et al. 2022). In addition to NO2-FAs drug-based treatments, dietary approaches that increase the endogenous levels of NO2-FAs are also promising. As dietary supplementation of conjugated linoleic acid (cLA) and 15N-labeled nitrite (NO2−) increased plasma nitro-conjugated linoleic acid (NO2-cLA) in healthy volunteers to levels that parallel concentrations attained in Phase I clinical studies of NO2-OA (Delmastro-Greenwood et al. 2015). These results support eating unsaturated fatty acids instead of saturated fatty acids and eating more green leafy vegetables or cereals, such as the Mediterranean diet, can help increase the level of NO2-FAs in the body and may reduce the risk of metabolic diseases.
NO2-FAs affect a variety of signaling pathways that are closely related to metabolic disease progression and are promising potential therapeutics for disease prevention. This review summarizes the production and metabolism of NO2-FAs, focusing on the mechanisms by which NO2-FAs exert protective effects toward diseases, such as CVDs, NAFLD, T2DM and obesity, and explores the therapeutic potential of NO2-FAs in these metabolic diseases.
2 Formation and metabolism of NO2-FAs
2.1 Formation of NO2-FAs
The most common NO2-FAs in the human body are NO2-OA, NO2-cLA, NO2-LA, and nitro linolenic acid (NO2-LNA) (Melo et al. 2019), likely because dietary nuts, meat, and dairy products are rich in PUFAs, such as oleic acid (OA), linoleic acid (LA), and cLA (Ros 2017). Several nitro-derivatives of other fatty acids have also been detected in animals, including nitro-arachidonic acid (NO2-AA), nitro-eicosapentaenoic acid (NO2-EPA), and nitro-docosahexaenoic acid (NO2-DHA) (Milic et al. 2015). In plants, NO2-FAs have been detected in Brassica napus, Pisum sativum, Oryza sativa, and fresh olives (Begara-Morales et al. 2021). These plants are considered an external source of NO2-FAs, and supplementing a diet with these foods may help to increase the endogenous levels of NO2-FAs (Begara-Morales et al. 2021; Fazzari et al. 2014). Upon eating nitrate-rich foods (green leafy vegetables, such as spinach and lettuce, and cauliflower and beetroot), the nitrates are reduced to nitrite in the oral cavity (Qin & Wang 2022), protonated in the gastric compartment, and combined with PUFAs from nuts, meat, and dairy products to generate NO2-FAs in humans and animals (Rocha et al. 2012). Clinical studies have indicated that when healthy volunteers received a diet supplemented with dietary nitrates and cLA, the levels of circulating NO2-FAs were increased (Delmastro-Greenwood et al. 2015). In addition to the digestive process, NO2-FAs are produced locally under conditions of inflammation and oxidative stress by the promotion of the nitration reaction between nitric oxide (NO)-derived oxidants and PUFAs (Radi 2018; Villacorta et al. 2018).
There are two mechanisms for the formation of NO2-FAs, depending on whether the compound contains a bis-allylic or conjugated configuration of double bonds. The main nitration agents are nitrogen oxide [NO, nitrogen dioxide (NO2)] radicals. The first mechanism involves the direct addition of NO2 to the double bond to form an alkyl radical, in which the intermediate undergoes cis/trans isomerization to form the final product. This process only occurs in the presence of relatively high NO2 concentrations. The second mechanism is typical for fatty acids (FAs) or lipids containing conjugated double bonds. The initial FA-radical is stabilized by resonance, which decreases the rate of the elimination reaction and favors reactions with NO2 or NO. The produced intermediates decompose to form the final nitroalkenes (Grippo et al. 2021). Nitration of PUFAs depends on the surrounding oxygen levels. At low oxygen concentrations (ischemic hypoxic conditions), nitration predominates, and at high oxygen concentrations, lipid peroxidation is the major pathway (Delmastro-Greenwood et al. 2014).
2.2 Metabolism and distribution of NO2-FAs
Most of the NO2-FAs produced in the stomach are absorbed by intestinal epithelial cells to form esterified NO2-FAs, which are subsequently assembled into chylomicrons in the mucosa of the small intestine, together with cholesterol esters, phospholipids, and apolipoproteins that are secreted extracellularly by the Golgi complex and then transported into the systemic circulation through the subclavian vein via lymphatic transport (Wit et al. 2022). Non-esterified NO2-FAs may be present as a consequence of absorption through the portal system and albumin-dependent transport (Brakenhielm & Alitalo 2019; Fazzari et al. 2019). Importantly, absorption through the lymphatic system avoids first-pass metabolism in the liver, greatly reducing the initial metabolism by phase I and II enzymes, and thus enhancing the oral bioavailability of NO2-FAs (Fazzari et al. 2019). Once the chylomicrons reach the capillaries, NO2-FAs will be stripped from the chylomicrons by lipoprotein lipase (LPL), and this process requires a docking protein glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) to anchor LPL to the endothelial lumen (Lundberg et al. 2018). In the vascular lumen, in addition to the NO2-FAs involved in the assembly of chylomicrons, approximately half NO2-FAs is added to albumin. The non-covalent binding of NO2-FAs with human serum albumin has been shown to occur at a molar ratio of 7:1 (Zatloukalova et al. 2019). In addition, NO2-FAs also easily undergo addition reactions with nucleophilic substances (such as thiol-containing proteins and glutathione). These addition reactions are reversible, and protein addition to NO2-FAs provides a reservoir of circulating NO2-FAs that has temporarily decreased electrophilic reactivity. The NO2-FAs can be subsequently released from the proteins (Hernychova et al. 2022; Rudolph et al. 2009). Therefore, the concentrations of free NO2-FAs in human plasma are very low. The free NO2-cLA concentrations in the plasma of 13 healthy human volunteers were determined to be 1–3 nM (Delmastro-Greenwood et al. 2015). Recently, researchers have examined the levels of NO2-OA and NO2-LA in healthy individuals and patients with ischemic heart disease. The NO2-OA and NO2 -LA levels in the plasma of 18 healthy volunteers were 12.6 ± 6 and 3.2 ± 1.7 nM, respectively, while the NO2-OA and NO2-LA levels in the plasma of 28 patients with ischemic heart disease were 21.7 ± 9.8 and 3 ± 1 nM, respectively (Herz et al. 2023).
Released NO2-FAs can bind to the fatty acid transporter CD36 to enter endothelial cells, or diffuse to target tissues, such as the heart, kidneys, liver, and adipose tissue. Once NO2-FAs reach the target cells, they are metabolized by various pathways through protein PTM involved in signaling pathways. The metabolites include (C2H4)n-shorter chain metabolites generated by mitochondrial β-oxidation, which are reduced by prostaglandin reductase 1 to non-electrophilic reactive nitroalkanes or esterified to form complex lipids (Fazzari et al. 2017). The accumulation of [14C]-NO2-OA in adipose tissue has indicated that adipocytes act as reservoirs as well as a buffering system for NO2-FAs (Fazzari et al. 2015). Preferential esterification of 10-NO2-OA has been shown to occur at the sn-2 position of triacylglycerides (Fazzari et al. 2019). These studies have indicated that the main mechanism for the tissue distribution of NO2-FAs involves complex lipid esterification, which helps to preserve the electrophilic properties of this PUFA derivatives, enabling efficient distribution to target organs. NO2-FAs are mobilized from adipocytes through adipotriglyceride lipase activity and transported back to the liver combined with albumin (Morigny et al. 2021). NO2-FAs reaching the liver can combine with triglycerides (TGs) and assemble into very low-density lipoprotein (VLDL) particles in the endoplasmic reticulum and Golgi compartment, which are released into the circulation and distributed systemically to target tissues together with mature VLDL particles containing apolipoproteins B, E, and CIII. This mobilization starts a cycle in which NO2-FAs participate in signal transduction, followed by metabolism and inactivation (Vitturi et al. 2013). Hydrophilic metabolites of NO2-FAs, including dicarboxylic acid derivatives, β-oxidation products, mercapturic acids, and cysteine adducts, are filtered in the kidneys and eliminated in the urine (Salvatore et al. 2021, 2013) (Fig. 1).
Biosynthesis and metabolism of NO2-FAs. a Dietary PUFAs and nitrates are ingested followed by nitrification in the stomach ventricles to generate NO2-FAs, which are absorbed by intestinal epithelial cells and assembled into chylomicrons, which enter the circulation through the lymphatic system. Esterified NO2-FAs or NO2-FAs combined with albumin are transported to various target organs for metabolism. Most NO2-FAs are excreted in the kidney. b After hydrolysis, esterified NO2-FAs are involved in cardiomyocyte, liver, fat, and renal regulatory signaling pathways, after cell entry via CD36. NO2-FAs, Nitro-fatty acids; PUFAs, polyunsaturated fatty acids; FA, fatty acids; GSNO2-18:1, glutathione (GSH)-adducted 18:1- NO2; TG, triglyceride; LPL, lipoprotein lipase; GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1; MRP, multidrug-resistant protein
3 Mechanism of action of NO2-FAs in metabolic diseases
Over the past decade, NO2-FAs have been shown to have a protective role in a variety of metabolic diseases in cell and animal models. This effect may be attributed to the involvement of NO2-FAs in signaling pathways that are anti-inflammatory or related to oxidative stress or that regulate metabolic pathways. The multiple mechanisms of action exerted by NO2-FAs in metabolic diseases and their therapeutic potential are summarized in Fig. 2 and Table 1.
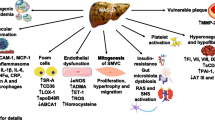
Potential mechanisms of action of NO2-FAs in disease. a In atherosclerotic cardiovascular diseases, NO2-FAs might reduce foam cell formation by inhibiting NF-κB, TLR4, ICAM-1, and VCAM-1, and inhibiting STAT-1 phosphorylation; activation of the Keap1/Nrf2 signaling pathway inhibits VSCMs proliferation to inhibit atherosclerosis. b In NAFLD, NO2-FAs might reverse lipid metabolism impairment, inflammation, and fibrosis enhancement by inhibiting the expression of the adipoietic genes SREBF1, MOGAT2, and SCD2, while downregulating genes involved in fatty acid β oxidation, including CPT2, HSD17B10, ACSL1, and PPARGC1A. c In T2DM, NO2-FAs might improve insulin resistance and glucose tolerance, and decrease blood glucose. d In cancer, NO2-FAs might induce caspase-depended apoptosis, mitochondrial dysfunction, and cancer cell arrest. e In nephropathy, NO2-FAs may reduce MMP levels by activating PPAR and Nrf2/Keap1, reducing blood glucose-preserving podocytes. The anti-inflammatory effects of combination therapy with losartan may be attributed to the ability of NO2-FAs to inhibit TNF-α and COX-2-mediated inflammatory pathways. f In obesity, NO2-FAs might decrease TG, free fatty acid, ROS and increase HDL in plasma by activating PPAR-γ and STING signal pathway. In addition, NO2-FAs might alter expression of the main triglyceride metabolizing enzymes DGAT1, HSL and ATGL. TLR4, Toll-likereceptor4; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; STAT-1, signal transducer and activator of transcription-1, Keap1, Kelch-like ECH-associated protein-1; Nrf2, nuclear factor-erythroid 2-related factor 2; VSCMs, vascular smooth muscle cells; NF-κB, nuclear factor kappa-B; NAFLD, non-alcoholic fatty liver disease; PPAR-γ, peroxisome proliferation-activated receptor gamma; TGF-β, transforming growth factor-β; ECM, extracellular matrix; MMP, matrix metalloproteinase; TNF-α, transforming growth factor-α; COX-2, cyclooxygenase-2; STING, stimulator of interferon genes; DGAT1, Diacylglycerol acyltransferase1; HSL, Hormone-sensitive lipase; ATGL, Adipose triglyceride lipase
3.1 CVDs
NO2-FAs, as endogenous products of cardiovascular system stress, were first identified in the mitochondria of cardiac ischemia-conditioned mice, and the potential reason may be that NO2 contributes to fatty acid nitrification under acidic conditions of ischemia/reperfusion (Nadtochiy et al. 2009). In C57BL/6J mice subjected to 30 min of coronary artery ligation, the formation of endogenous NO2-OA and NO2-LA was observed after 30 min of reperfusion, whereas no NO2-FAs were detected in sham-operated mice or in mice with myocardial infarction without reperfusion (Rudolph et al. 2010a, b). Subsequently, in more cardiovascular disease studies, it was found that NO2-FAs participate in the regulation of a wide range of signaling pathways related to inflammation, oxidative stress, and glucose and lipid metabolism through PTM modification to exert cardiovascular protective effects (Mollenhauer et al. 2018). Here, we summarize the studies related to NO2-FAs and CVDs (Table 1).
3.1.1 Atherosclerosis
Atherosclerosis, a major cause of CVDs, is characterized by chronic inflammation and excessive lipid deposition in the innermost layer of the arteries (Björkegren & Lusis 2022; McAlpine & Swirski 2016). Multiple molecular mechanisms may be involved in the anti-inflammatory effects of NO2-FAs. For example, NO2-OA and NO2-LA can covalently bind to the p65 subunit of NF-κB, inhibiting DNA binding activity, thus, repressing NF-κB-dependent gene expression; inhibiting the secretion of the pro-inflammatory factor interleukin (IL)-6, tumor necrosis factor α (TNFα), monocyte chemoattraction protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1) in downstream macrophages; and inhibiting the adherence of monocytes to endothelial cells (Cui et al. 2006). Intravenous injection of NO2-OA in a lipopolysaccharide (LPS)-induced NF-kB-luciferase transgenic mouse model of inflammation, indicated that nanomolar levels of NO2-OA in the plasma can reduce vascular inflammatory responses in vivo. In vitro experiments have shown that NO2-OA can suppress LPS-induced TLR4 signaling in lipid rafts and inhibit the pro-inflammatory activation of the NF-κB upstream signal IκB/IKK (Villacorta et al. 2013). In addition, NO2-OA and NO2-LA inhibited proinflammatory signal transducer and STAT signaling through inducing mitogen-activated protein kinase phosphatase 1 (MKP-1) in macrophages (Ichikawa et al. 2008). Similarly, both NO2-OA and NO2-LA prevented the TNFα-stimulated release of inflammatory factors, such as IL-6, IL-8, and IL-12, from endothelial cells, and blocked TNFα-induced expression of intercellular cell adhesion molecule-1 (ICAM-1) (Hwang et al. 2009). Subcutaneous administration of 9-NO2-OA and 10-NO2-OA for 3 weeks (8 mg/kg/day) effectively reduced atherosclerotic lesion formation in apolipoprotein E deficient (apoE−/−) mice. In addition to reducing the number of inflammatory cells and the expression of inflammatory factors, NO2-OA reduced the phosphorylation of the activator of STAT-1 induced by oxidized low-density lipoprotein (LDL) to reduce the formation of foam cells (the characteristic cells of atherosclerosis) (Rudolph et al. 2010a, b).
Endothelial injury is a key step in the initiation of atherosclerosis (Björkegren & Lusis 2022). Recently, transcriptome analysis of human coronary endothelial cells has indicated that NO2-FAs exert a protective effect by regulating hypoxia and antioxidant-related pathways. NO2-cLA modulated hypoxia responses by increasing the expression of angiopoietin-like 4 (ANGPTL4) in endothelial cells (Lu et al. 2019), and ANGPTL4 can promote angiogenesis and prevent myocardial infarction (Galaup et al. 2012; Stitziel et al. 2016). In addition, NO2-cLA is not only a potent inducer of the antioxidant genes HMOX1 and NQO1, but also a repressor of the pro-oxidative gene NADPH oxidase 4, highlighting the critical role of NO2-cLA in maintaining redox homeostasis in endothelial cells (Lu et al. 2019). Notably, NO2-LA is a potent inducer of heme oxygenase 1 (HO-1) gene expression, a central defensive enzyme in tissue anti-inflammatory responses to vascular injury (Zhang et al. 2021), which contributes to the inhibition of atherosclerosis. The induction of HO-1 by NO2-LA far exceeded that induced by equimolar concentrations of LA or oxidized LA (Wright et al. 2006).
Lipid metabolism is key to atherosclerosis (Mehta & Shapiro 2022), and NO2-FAs have been found to regulate lipid metabolism in cells. The uptake of oxidatively modified low-density lipoprotein (mLDL) by macrophages leading to its excessive accumulation in the cytoplasm plays a key role in the early stages of atherosclerosis development (Moore & Tabas 2011). CD36 promotes the binding and uptake of long-chain FAs and mLDL into cells (Shu et al. 2022). NO2-FAs were found to be powerful activators of PPAR-γ in 2005. The activation of PPAR-γ by NO2-FAs induced PPAR-γ-dependent macrophage CD36 expression and adipocyte differentiation and glucose uptake, and the activation potency was comparable to that of the classic PPAR-γ activators, thiazolidinediones (TZDs) (Baker et al. 2005; Schopfer et al. 2005). Later studies found that NO2-OA exerted an anti-atherosclerotic effect by reducing the TG content of macrophages (Rosenblat et al. 2016). Ligand binding analysis showed that a specific interaction between NO2-OA and Lys164 in CD36 restricted the binding and uptake of mLDL by CD36. In addition, NO2-OA restored the autophagic flux of mLDL-loaded macrophages, thereby reversing the deposition of mLDL cholesterol in cells (Vazquez et al. 2020). Recent experiments determined that the average level of apolipoproteinB-100 containing NO2-cLA was 5 pmol/mg in human LDL samples, which was approximately 10% of the content of total cLA (free and esterified) on LDL (Mastrogiovanni et al. 2020). This suggests that under specific circumstances, NO2-FAs-loaded LDL may be formed endogenously and exert a protective effect by releasing NO2-FAs. In the course of the development of atherosclerosis lesions, vascular smooth muscle cells (VSCMs) transition from a contractile state to a proliferative state and migrate to the inner membrane. Proliferative differentiated macrophage-like VSMCs absorb lipid-producing foam cells (Basatemur et al. 2019; Pan et al. 2020). NO2-LA arrested the growth of VSMCs in the G1/S phase of the cell cycle by up-regulating the cyclin-dependent kinase inhibitor p27kip1 via the Keap1/Nrf2 pathway (Villacorta et al. 2007). Transcriptome analysis showed that NO2-FAs inhibited the proliferation of human coronary artery smooth muscle cells (hCASMC), and antioxidant defense was also the main regulation of NO2-cLA to hCASMC. External stimuli, such as DNA damaging agents or oxidative stress, can cause stress-induced cell growth arrest (Grootaert & Bennett 2021). Compared with cLA-treated or control groups, NO2-cLA-treated groups showed upregulated key factors induced by oxidative stress, including HMOX1, GCLM, GCLC, TXNRD1, and SLC7A11. This study also revealed novel gene targets in key pathways by which NO2-cLA regulated lipid metabolism and inflammation in hCASMC, including perilipin-2 (PLIN2) related to lipid storage and highly expressed macrophage migration inhibitory factor (MIF) (Li et al. 2018). These results indicated that the influence of NO2-FAs on VSCMs was multifaceted and is worthy of further exploration. Taken together, these results indicated that NO2-FAs participate in multiple signaling events to promote overall atherosclerosis protection (Fig. 3).
The main biological effects of NO2-FAs on the cardiovascular system and the main corresponding cellular signaling pathways. NO2-FAs act as lipid mediators eliciting numerous biological responses that can impact both vascular and cardiac function, including lipid metabolism, mild decoupling, anti-inflammatory, vasodilation, elastin fragmentation, antifibrosis, and apoptosis effects
3.1.2 Hypertension
Hypertension is one of the most important modifiable metabolic risk factors for CVDs and one of the leading causes of morbidity and mortality worldwide (Brouwers et al. 2021). In a mouse model of angiotensin II (AngII)-induced hypertension, infusion of NO2-OA instead of OA lowered blood pressure and inhibited vasoconstriction. Even with pre-existing hypertension, NO2-OA-treated mice showed a significant reduction compared to controls in blood pressure (Zhang et al. 2010). Another study demonstrated that NO2-OA reduced AngII-induced hypertension and inhibited cardiac hypertrophy by inhibiting sEH, which prevented epoxyeicosatrienoic acid (EET) hydrolysis of sEH substrates, inducing vasodilation to lower the blood pressure after EET accumulation (Charles et al. 2014). Mechanistically, NO2-OA inhibited AngII-mediated vasoconstriction by binding to the AngII receptor, AT1R, but NO2-OA did not affect the binding of AngII with the AT1R. NO2-OA interfered with the coupling of G-protein signaling and calcium mobilization downstream of the AT1R, thereby inhibiting VSCMs contraction (Zhang et al. 2010). NO2-OA binds to Cys521 near the center of sEH, leading to the decrease of the hydrolase activity and inhibiting the metabolism of EET to the corresponding diol or dihydroxyeicosatrienoic acid. Therefore, NO2-OA could not abrogate AngII-induced hypertension and cardiac hypertrophy in Cys521Ser sEH redox knock-in mice (Charles et al. 2014). Human sEH (hsEH) is a therapeutic target, but no approved drugs are available. Recent biochemical and biophysical studies have shown that 9-NO2-OA, 10-NO2-OA, and 10-NO2-LA can not only bind to the Cys423 and Cys522 (equivalent to mouse Cys521) of hsEH, but can also bind covalently to other nucleophilic residues in the sEH C-terminal domain (Qiu et al. 2022). Utilizing this binding may provide an alternative pharmacological approach for sEH drugs, which have shown disappointing results in clinical trials to date. In addition, it is worth investigating whether the direct consumption of NO2-FAs can reduce blood pressure.
3.1.3 Arrhythmia
The occurrence of ventricular arrhythmias, such as sustained ventricular tachycardia (VT), most often leads to AMI-related sudden cardiac death (Kosmidou et al. 2017). Cardiac metabolic disorders and redox state abnormalities during heart disease stimulate arrhythmogenic substrates by directly or indirectly modulating cardiac ion channel/transporter function (Yang et al. 2015). Ca2+ plays an important role in maintaining the excitation–contraction coupling and electrical rhythm of the normal heart, and abnormalities in Ca2+ homeostasis play a key role in the pathogenesis of common CVDs, including arrhythmia (Landstrom et al. 2017). The pretreatment of mice with NO2-OA significantly reduced the susceptibility to acute VT. NO2-OA attenuated RyR2-dependent Ca2+ leakage of calmodulin-dependent kinase II (CaMKII) activity and prevented VT after AMI (Mollenhauer et al. 2020). By inhibiting CaMKII, NO2-OA regulated key pathways of electrical remodeling after AMI. Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with an increased risk of death worldwide, and AF does not respond well to current treatments (Brundel et al. 2022). Fibrosis is one of the most prominent features of AF pathology, and inflammation promotes cardiomyocyte fibrosis (Burstein & Nattel 2008; Rockey et al. 2015). In vivo, NO2-FAs administration effectively reduced atrial fiber remodeling in AngII-pretreated mice, as well as reducing AF susceptibility and atrial conduction inhomogeneity. These effects of NO2-FAs were mediated by the inhibition of Smad2-dependent myofibroblast transdifferentiation, as well as the inhibition of oxidative inflammatory mediators of atrial production (Rudolph et al. 2016). In another study, NO2-OA treatment attenuated interstitial myocardial fibrosis and greatly improved left ventricular systolic function in mice with dilated cardiomyopathy. In vitro studies further indicated that the anti-fibrotic effect of NO2-OA depended on inhibiting the phosphorylation of downstream targets of transforming growth factor-β (TGF-β) to weaken the ability of fibroblasts to transform into myofibroblasts (Braumann et al. 2021) (Fig. 3). NO2-FAs thus emerge as potential therapeutic agents for ventricular arrhythmias either by increasing endogenous levels through dietary interventions or as synthetic drugs.
3.1.4 Aortic aneurysm
Thoracic aortic aneurysms occur primarily in the patient’s aortic root and ascending aorta, with a potential risk of rupture that could be life-threatening to the patient (Pirruccello et al. 2022). Marfan syndrome (MFS) is a relatively common genetic disorder of connective tissue that can progress to thoracic aortic aneurysm (Milewicz et al. 2021). Standard medical treatment for MFS includes β-receptor blocker therapy (Hiratzka et al. 2010), which slows the rate of aortic dilation in patients with MFS (Shores et al. 1994). Although several potential drug targets have been identified in animal studies, no therapeutic agents have been identified that can prevent aneurysm formation in MFS (Milewicz & Ramirez 2019). With the increasing prevalence of hypertension and atherosclerosis, morbidity and mortality caused by abdominal aortic aneurysm (AAA) have gradually increased, resulting in a serious medical burden for individuals and society (Yu et al. 2022). There is currently no strong scientific evidence that drug therapy can reduce the growth of AAAs, which is because of the complex etiology of AAAs (Sakalihasan et al. 2018). The induction of elastin fragmentation and the apoptosis of smooth muscle cells is the main mechanism driving the aortic lesions of MFS (Milewicz et al. 2021). AAAs arise from structural changes in the aortic wall, including media and adventitia thinning because of the loss of VSCMs and degradation of the extracellular matrix (Sakalihasan et al. 2018). MFS mice were used in a thoracic aortic aneurysm model (Nettersheim et al. 2022) and AAV. PCSK9-D377Y-induced hypercholesterolemia combined with chronic infusion of Ang II was used to induce the formation of an AAA in another mouse model (Zhao et al. 2019).
Another study investigated a nitroalkene-Trolox™ derivative named NATx0 as an unconventional anti-inflammatory strategy to treat chronic inflammatory diseases, such as obesity-induced glucose intolerance. NATx0 inhibited NF-kB nuclear translocation and pro-inflammatory gene expression in macrophages in vitro. Furthermore, NATx0 treatment prevented NLRP3 inflammasome activation following macrophage LPS/ATP stimulation in vitro. When tested acutely in vivo, NATx0 inhibited neutrophil recruitment in zebrafish larvae and reduced IL-1β production after LPS challenge in mice. Long-term administration of NATx0 in diet-induced obese mice reduced muscle tissue inflammation and glucose intolerance, thereby improving glucose homeostasis. The nitroalkene-Trolox™ derivative was found to be a suitable tool to address acute and chronic inflammation in vitro and in vivo, mainly through the inhibition of NF-kB/NLRP3 activation (Dapueto et al. 2021). These studies demonstrated that this pharmacological strategy, using unconventional anti-inflammatory compounds and a safe drug delivery system, has great potential for clinical application (Table 2).
4.5 Supplement with unsaturated fatty acids and nitrates
Diet may contribute to disease risk through modulation of metabolic pathways and homeostasis (Shoaie et al. 2015). Differences in metabolic responses to diet may explain some individual variations in diet disease associations (Li et al. 2020). The 2015–2020 Dietary Guidelines state that the Mediterranean diet is recommended as an important and cost-effective strategy for the prevention of CVDs (Li et al. 2020). The Mediterranean diet is characterized by a high consumption of fruits, vegetables, seafood, nuts, legumes, whole grains, and olive oil, a moderate intake of wine primarily in the diet, a reduced intake of red/processed meats, saturated fats, and sugary desserts and beverages (Benjamin et al. 2017). It can be seen that the Mediterranean diet includes more unsaturated fatty acids and proteins, and a moderate amount of carbohydrates (Delgado-Lista et al. 2022). Dietary vegetables will produce nitrite through bacterial action in the mouth. Therefore, Mediterranean diet favors the formation of endogenous NO2-FAs in the body.
In healthy volunteers, the plasma concentration of 15NO2-cLA (Cmax 8 nM) achieved by cLA and 15NO2− supplementation was consistent with the NO2-OA concentration at the defined target dose when a Phase II clinical trial (pulmonary hypertension, chronic kidney disease, and asthma, 150 mg dose, Cmax 7.6 nM) was conducted (NCT02248051, NCT03449524, NCT03422510). In a mouse model of myocardial infarction, oral treatment with HNO2 and cLA protects cardiac function and prevents myocardial hypertrophy by increasing cardioprotective microRNA-499 levels and subsequently decreasing p53 and dynamin-related protein 1 (DRP-1) expression. The underlying mechanism may be that cLA and HNO2 produce NO2-cLA in vivo and are involved in mitochondrial function and apoptosis pathways (Qipshidze-Kelm et al. 2013). Supplementing with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) may reduce the risk of cardiovascular disease (Rodriguez et al. 2022). However, the results of randomized controlled trials investigating the effects of omega-3 supplementation on CVDs risk have been inconsistent (Sherratt et al. 2023), not everyone reduces TG levels through omega-3 supplementation. Different participant characteristics may contribute to this inter-individual variation in TG response (Rundblad et al. 2023). It is very interesting to see whether the level of NO2-FAs as derivatives of unsaturated fatty acids in the human body can further explain the results of clinical trials of these unsaturated fatty acids.
5 Conclusions and future directions
In recent years, with advances in detection technology and animal disease models, a deeper understanding of the protective mechanism of NO2-FAs has been established. Here, we summarized the mechanism of action and therapeutic potential of NO2-FAs in various diseases. At present, research into the potential mechanisms for the extensive pharmacological effects of NO2-FAs has mainly focused on the regulation of signaling pathways, such as NF-κB, STAT, NADPH, PPAR-γ, and Keap1/Nrf2. However, use of emerging omics analysis techniques continues to reveal new cellular targets for NO2-FAs (Fang et al. 2021; Xu et al. 2023). Transcriptome data, coupled with lipidomic, proteomic- and MS-based approaches, will help prioritize and guide which pathways to pursue. In addition, the “one drug-multiple targets” feature of NO2-FAs may provide new therapeutic strategies for diseases, especially for diseases showing multifactorial pathogenesis. NO2-FAs exist in a variety of natural plants and edible oils; therefore, increasing the dietary intake of these foods may help to increase the levels of NO2-FAs in the human body. The Mediterranean diet is characterized by a high intake of unsaturated fatty acids, especially olive oil and fish rich in OA and LA, and vegetables containing NO2– and NO32–. The acidic and hypoxic conditions in the stomach provide an environment for the efficient nitration of unsaturated fatty acids by nitrite. Several plausible mechanisms have been proposed to explain the epidemiological evidence for the benefits of a Mediterranean-style diet; however, some of the findings mentioned earlier indicate that the consumption of unsaturated fats and vegetables rich in nitrites and nitrates can provide protection against some diseases. In addition, absorption, distribution, metabolism, and excretion studies of radiolabeled CXA-10 in rodents has demonstrated an oral bioavailability of up to 35%. Nano-targeted delivery technology may be used to develop an effective way to improve the bioavailability of NO2-FAs, which may provide avenues for further development and utilization. Great attention should be paid to the combined therapeutic effects and underlying mechanisms of NO2-FAs in combination with proven drugs in the treatment of certain diseases.
However, there is still much to be elucidated to better use NO2-FAs to enhance the health benefits in humans. In recent years, several NO2-FAs have been identified in in biological samples, and the concentrations determined by liquid chromatography-mass spectrometry and gas chromatography-chromatography-mass spectrometry techniques. Several NO2-FAs have been identified and quantified, including NO2-OA and NO2-LA, in the plasma of healthy and ill subjects with concentrations over three orders of magnitude, i.e., concentrations from several hundred pM to several hundred nM. These extremely broad concentration ranges indicate serious chromatographic and mass spectrometric deficiencies, including preanalytical issues such as artifacts and contamination (Schopfer et al. 2014; Tsikas 2023). Challenges in detection technologies have limited the analysis of correlations between NO2-FAs and clinical metabolic diseases. However, in the Phase I clinical trial of sodium nitrite for out of hospital cardiac arrest (NCT02987088), survivors of cardiac arrest that had higher levels of NO2-FAs had less neurological damage (Vitturi et al. 2020). This result appears to indicate a better prognosis for patients with high levels of NO2-FAs. In addition, a statistically significant difference in the concentrations of NO2-OA in the plasma of patients with myocardial infarction compared with those in the plasma of healthy volunteers was preliminary observed (Herz et al. 2023). This result indicated that NO2-FAs levels in the body may be a marker of the patient’s ability to respond to anti-inflammatory and oxidative stress, however, this needs to be validated in a larger cohort. Further clinical research into the treatment of metabolic diseases using NO2-FAs is required. In addition, NO2-FAs have a wide range of pharmacological effects, not limited to a specific disease, and it may be possible to develop NO2-FAs as drug adjuvants.
This article reviews the mechanisms by which NO2-FAs exert protective effects in various metabolic diseases and the therapeutic potential of NO2-FAs. Studies conducted over the past 20 years have revealed that NO2-FAs can alleviate inflammatory and oxidative stress damage in metabolic diseases by targeting the related signaling pathways (such as NF-κB, STAT, NADPH, PPAR-γ, and Keap1/Nrf2) and, thus, exert cardiopulmonary vascular protective, neuroprotective, hepatoprotective, renal protective, skin protective, anti-diabetic, lipid lowing, and anti-cancer effects. Overall, NO2-FAs are active lipid molecules with great therapeutic potential that may be also developed into nutritional supplements.
Availability of data and materials
NA.
References
Adhyaru, Jacobson. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757–69. https://doi.org/10.1038/s41569-018-0098-5.
Arbeeny, Ling, Smith, O’Brien, Wawersik, Ledbetter, et al. CXA-10, a nitrated fatty acid, is renoprotective in deoxycorticosterone acetate-salt nephropathy. J Pharmacol Exp Ther. 2019;369(3):503–10. https://doi.org/10.1124/jpet.118.254755.
Asan, Skoko, Woodcock, Wingert, Woodcock, Normolle, et al. Electrophilic fatty acids impair RAD51 function and potentiate the effects of DNA-damaging agents on growth of triple-negative breast cells. J Biol Chem. 2019;294(2):397–404. https://doi.org/10.1074/jbc.AC118.005899.
Awwad, Steinbrink, Frömel, Lill, Isaak, Häfner, et al. Electrophilic fatty acid species inhibit 5-lipoxygenase and attenuate sepsis-induced pulmonary inflammation. Antioxid Redox Signal. 2014;20(17):2667–80. https://doi.org/10.1089/ars.2013.5473.
Baker, Lin, Schopfer, Woodcock, Groeger, Batthyany, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280(51):42464–75. https://doi.org/10.1074/jbc.M504212200.
Ban, Twigg, Franjic, Brooks, Celermajer, Yue, et al. Serum MMP-7 is increased in diabetic renal disease and diabetic diastolic dysfunction. Diabetes Res Clin Pract. 2010;87(3):335–41. https://doi.org/10.1016/j.diabres.2010.01.004.
Bansal G, Thanikachalam PV, Maurya RK, Chawla P, Ramamurthy S. An overview on medicinal perspective of thiazolidine-2,4-dione: a remarkable scaffold in the treatment of type 2 diabetes. J Adv Res. 2020;23:163–205. https://doi.org/10.1016/j.jare.2020.01.008.
Basatemur, Jørgensen, Clarke, Bennett, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–44. https://doi.org/10.1038/s41569-019-0227-9.
Bedard, Krause. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. https://doi.org/10.1152/physrev.00044.2005.
Begara-Morales, Mata-Pérez, Padilla, Chaki, Valderrama, Aranda-Caño, et al. Role of electrophilic nitrated fatty acids during development and response to abiotic stress processes in plants. J Exp Bot. 2021;72(3):917–27. https://doi.org/10.1093/jxb/eraa517.
Begriche, Massart, Robin, Bonnet, Fromenty. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58(4):1497–507. https://doi.org/10.1002/hep.26226.
Benjamin, Blaha, Chiuve, Cushman, Das, Deo, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603. https://doi.org/10.1161/cir.0000000000000485.
Bhatt, Steg, Miller, Brinton, Jacobson, Ketchum, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. https://doi.org/10.1056/NEJMoa1812792.
Björkegren, Lusis. Atherosclerosis: recent developments. Cell. 2022;185(10):1630–45. https://doi.org/10.1016/j.cell.2022.04.004.
Bonacci, Schopfer, Batthyany, Rudolph, Rudolph, Khoo, et al. Electrophilic fatty acids regulate matrix metalloproteinase activity and expression. J Biol Chem. 2011;286(18):16074–81. https://doi.org/10.1074/jbc.M111.225029.
Brakenhielm, Alitalo. Cardiac lymphatics in health and disease. Nat Rev Cardiol. 2019;16(1):56–68. https://doi.org/10.1038/s41569-018-0087-8.
Braumann, Schumacher, Im, Nettersheim, Mehrkens, Bokredenghel, et al. Nitro-Oleic Acid (NO(2)-OA) improves systolic function in dilated cardiomyopathy by attenuating myocardial fibrosis. Int J Mol Sci. 2021;22(16):9052. https://doi.org/10.3390/ijms22169052.
Brouwers, Sudano, Kokubo, Sulaica. Arterial hypertension. Lancet. 2021;398(10296):249–61. https://doi.org/10.1016/s0140-6736(21)00221-x.
Brundel, Ai, Hills, Kuipers, Lip, de Groot. Atrial fibrillation. Nat Rev Dis Primers. 2022;8(1):21. https://doi.org/10.1038/s41572-022-00347-9.
Burstein, Nattel. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–9. https://doi.org/10.1016/j.jacc.2007.09.064.
Carlström. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat Rev Nephrol. 2021;17(9):575–90. https://doi.org/10.1038/s41581-021-00429-z.
Chalasani, Younossi, Lavine, Diehl, Brunt, Cusi, et al The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. https://doi.org/10.1053/j.gastro.2012.04.001.
Charles, Rudyk, Prysyazhna, Kamynina, Yang, Morisseau, et al. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2014;111(22):8167–72. https://doi.org/10.1073/pnas.1402965111.
Chew, Ng, Tan, Kong, Lin, Chin, et al. The global burden of metabolic disease: data from 2000 to 2019. Cell Metab. 2023;35(3):414-428.e413. https://doi.org/10.1016/j.cmet.2023.02.003.
Cui, Schopfer, Zhang, Chen, Ichikawa, Baker, et al. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281(47):35686–98. https://doi.org/10.1074/jbc.M603357200.
Dapueto, Rodriguez-Duarte, Galliussi, Kamaid, Bresque, Batthyány, et al. A novel nitroalkene vitamin E analogue inhibits the NLRP3 inflammasome and protects against inflammation and glucose intolerance triggered by obesity. Redox Biol. 2021;39:101833. https://doi.org/10.1016/j.redox.2020.101833.
Delgado-Lista, Alcala-Diaz, Torres-Peña, Quintana-Navarro, Fuentes, Garcia-Rios, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399(10338):1876–85. https://doi.org/10.1016/s0140-6736(22)00122-2.
Delmastro-Greenwood, Freeman, Wendell. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol. 2014;76:79–105. https://doi.org/10.1146/annurev-physiol-021113-170341.
Delmastro-Greenwood, Hughan, Vitturi, Salvatore, Grimes, Potti, et al. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic Biol Med. 2015;89:333–41. https://doi.org/10.1016/j.freeradbiomed.2015.07.149.
DeMarsilis, Reddy, Boutari, Filippaios, Sternthal, Katsiki, et al. Pharmacotherapy of type 2 diabetes: An update and future directions. Metabolism. 2022;137155332. https://doi.org/10.1016/j.metabol.2022.155332.
Di Cesare, Di Meglio, Nestle. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–50. https://doi.org/10.1038/jid.2009.59.
Di Maio, Keeney, Cechova, Mortimer, Sekandari, Rowart, et al. Neuroprotective actions of a fatty acid nitroalkene in Parkinson’s disease. NPJ Parkinsons Dis. 2023;9(1):55. https://doi.org/10.1038/s41531-023-00502-3.
Diaz-Amarilla, Miquel, Trostchansky, Trias, Ferreira, Freeman, et al. Electrophilic nitro-fatty acids prevent astrocyte-mediated toxicity to motor neurons in a cell model of familial amyotrophic lateral sclerosis via nuclear factor erythroid 2-related factor activation. Free Radic Biol Med. 2016;95:112–20. https://doi.org/10.1016/j.freeradbiomed.2016.03.013.
Elmaleh-Sachs, Schwartz, Bramante, Nicklas, Gudzune, Jay. Obesity management in adults: a review. JAMA. 2023;330(20):2000–15. https://doi.org/10.1001/jama.2023.19897.
Fang, Huang, Tu, Chen, Pan, Hsiao, et al. Chemoproteomic profiling reveals cellular targets of nitro-fatty acids. Redox Biol. 2021;46:102126. https://doi.org/10.1016/j.redox.2021.102126.
Fazzari, Trostchansky, Schopfer, Salvatore, Sánchez-Calvo, Vitturi, et al. Olives and olive oil are sources of electrophilic fatty acid nitroalkenes. PLoS One. 2014;9(1):e84884. https://doi.org/10.1371/journal.pone.0084884.
Fazzari, Khoo, Woodcock, Li, Freeman, Schopfer. Generation and esterification of electrophilic fatty acid nitroalkenes in triacylglycerides. Free Radic Biol Med. 2015;87:113–24. https://doi.org/10.1016/j.freeradbiomed.2015.05.033.
Fazzari, Khoo, Woodcock, Jorkasky, Li, Schopfer, Freeman. Nitro-fatty acid pharmacokinetics in the adipose tissue compartment. J Lipid Res. 2017;58(2):375–85. https://doi.org/10.1194/jlr.M072058.
Fazzari, Vitturi, Woodcock, Salvatore, Freeman, Schopfer. Electrophilic fatty acid nitroalkenes are systemically transported and distributed upon esterification to complex lipids. J Lipid Res. 2019;60(2):388–99. https://doi.org/10.1194/jlr.M088815.
Foulkes, Smith, Reis-Filho. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. https://doi.org/10.1056/NEJMra1001389.
Fraga, Trostchansky, Rocha, Laran**ha, Rubbo, Galleano. (Poly)phenols and nitrolipids: relevant participants in nitric oxide metabolism. Mol Aspects Med. 2023;89:101158. https://doi.org/10.1016/j.mam.2022.101158.
Fu, Schoeman, Harms, van Wietmarschen, Vreeken, Berger A, Cuppen, et al. Metabolomics profiling of the free and total oxidised lipids in urine by LC-MS/MS: application in patients with rheumatoid arthritis. Anal Bioanal Chem. 2016;408(23):6307–19. https://doi.org/10.1007/s00216-016-9742-2.
Gadde, Martin, Berthoud, Heymsfield. Obesity: Pathophysiology and Management. J Am Coll Cardiol. 2018;71(1):69–84. https://doi.org/10.1016/j.jacc.2017.11.011.
Galaup, Gomez, Souktani, Durand, Cazes, Monnot, et al. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation. 2012;125(1):140–9. https://doi.org/10.1161/circulationaha.111.049072.
Garner A, Mould A, Chieffo A, Jorkasky A. Pharmacokinetic and pharmacodynamic effects of Oral CXA-10, a nitro fatty acid, after single and multiple ascending doses in healthy and obese subjects. Clin Transl Sci. 2019;12(6):667–76. https://doi.org/10.1111/cts.12672.
Green, Maceyka, Cowart, Spiegel. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021;33(7):1293–306. https://doi.org/10.1016/j.cmet.2021.06.006.
Greten, Grivennikov. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. https://doi.org/10.1016/j.immuni.2019.06.025.
Grippo, Mojovic, Pavicevic, Kabelac, Hubatka, Turanek, et al. Electrophilic characteristics and aqueous behavior of fatty acid nitroalkenes. Redox Biol. 2021;38:101756. https://doi.org/10.1016/j.redox.2020.101756.
Grootaert, Bennett. Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc Res. 2021;117(11):2326–39. https://doi.org/10.1093/cvr/cvab046.
Grundy, Stone, Bailey, Beam, Birtcher, Blumenthal, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143. https://doi.org/10.1161/cir.0000000000000625.
Guo, Wang, Chen, Xu. Functions of amyloid precursor protein in metabolic diseases. Metabolism. 2021;115:154454. https://doi.org/10.1016/j.metabol.2020.154454.
Haas, Francque, Staels. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol. 2016;78:181–205. https://doi.org/10.1146/annurev-physiol-021115-105331.
Halade, Black, Verma. Paradigm shift - metabolic transformation of docosahexaenoic and eicosapentaenoic acids to bioactives exemplify the promise of fatty acid drug discovery. Biotechnol Adv. 2018;36(4):935–53. https://doi.org/10.1016/j.biotechadv.2018.02.014.
Hansen, Buchan, Rühl, Mukai, Salvatore, Ogawa, et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc Natl Acad Sci U S A. 2018;115(33):E7768–75. https://doi.org/10.1073/pnas.1806239115.
Hellmuth, Brat, Awad, George, Kahnt, Bauer, et al. Structural modifications yield novel insights into the intriguing pharmacodynamic potential of anti-inflammatory nitro-fatty acids. Front Pharmacol. 2021;12:715076. https://doi.org/10.3389/fphar.2021.715076.
Hernychova, Alexandri, Tzakos, Zatloukalová, Primikyri, Gerothanassis, et al. Serum albumin as a primary non-covalent binding protein for nitro-oleic acid. Int J Biol Macromol. 2022;203:116–29. https://doi.org/10.1016/j.ijbiomac.2022.01.050.
Herz, Gad, Hanafi. Development and validation of a bioanalytical method for the quantification of nitrated fatty acids in plasma using LC-MS/MS: application to cardiovascular patients. Separations. 2023;10(2):87.
Hiratzka, Bakris, Beckman, Bersin, Carr, Casey, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266–369. https://doi.org/10.1161/CIR.0b013e3181d4739e.
Hwang, Lee, Lim, Park. Nitrated fatty acids prevent TNFalpha-stimulated inflammatory and atherogenic responses in endothelial cells. Biochem Biophys Res Commun. 2009;387(4):633–40. https://doi.org/10.1016/j.bbrc.2009.07.030.
Ichikawa, Zhang, Chen, Liu, Schopfer, Baker, et al. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology. 2008;149(8):4086–94. https://doi.org/10.1210/en.2007-1639.
Kansanen, Kuosmanen, Ruotsalainen, Hynynen, Levonen. Nitro-oleic acid regulates endothelin signaling in human endothelial cells. Mol Pharmacol. 2017;92(4):481–90. https://doi.org/10.1124/mol.117.109751.
Kelley, Baust, Bonacci, Golin-Bisello, Devlin, St Croix, et al. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res. 2014;101(3):352–63. https://doi.org/10.1093/cvr/cvt341.
Khoo, Li, Salvatore, Schopfer, Freeman. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-κB signaling: a medicinal chemistry investigation of structure-function relationships. Sci Rep. 2018;8(1):2295. https://doi.org/10.1038/s41598-018-20460-8.
Khoo, Fazzari, Chartoumpekis, Li, Guimaraes, Arteel, et al. Electrophilic nitro-oleic acid reverses obesity-induced hepatic steatosis. Redox Biol. 2019;22:101132. https://doi.org/10.1016/j.redox.2019.101132.
Khunti, Chatterjee, Gerstein, Zoungas, Davies. Do sulphonylureas still have a place in clinical practice? Lancet Diabetes Endocrinol. 2018;6(10):821–32. https://doi.org/10.1016/s2213-8587(18)30025-1.
Kosmidou, Embacher, McAndrew, Dizon, Mehran, Ben-Yehuda, Mintz, et al. Early ventricular tachycardia or fibrillation in patients with ST Elevation myocardial infarction undergoing primary percutaneous coronary intervention and impact on mortality and stent thrombosis (from the harmonizing outcomes with revascularization and stents in acute myocardial infarction trial). Am J Cardiol. 2017.120(10):1755–60. https://doi.org/10.1016/j.amjcard.2017.07.080.
Koutoulogenis A, Kokotos A. Nitro Fatty Acids (NO(2)-FAs): an emerging class of bioactive fatty acids. Molecules. 2021;26(24). https://doi.org/10.3390/molecules26247536.
Kraakman, Liu, Postigo-Fernandez, Ji, Kon, Larrea, et al. PPARγ deacetylation dissociates thiazolidinedione's metabolic benefits from its adverse effects. J Clin Invest. 2018;128(6):2600–12. https://doi.org/10.1172/jci98709.
Kühn, Brat, Fettel, Hellmuth, Maucher, Bulut, et al. Anti-inflammatory nitro-fatty acids suppress tumor growth by triggering mitochondrial dysfunction and activation of the intrinsic apoptotic pathway in colorectal cancer cells. Biochem Pharmacol. 2018;155:48–60. https://doi.org/10.1016/j.bcp.2018.06.014.
Landstrom, Dobrev, Wehrens. Calcium signaling and cardiac arrhythmias. Circ Res. 2017.120(12):1969–1993. https://doi.org/10.1161/circresaha.117.310083.
Li, Chang, Zhu, Villacorta, Li, Freeman, et al. Transcriptomic sequencing reveals diverse adaptive gene expression responses of human vascular smooth muscle cells to nitro-conjugated linoleic acid. Physiol Genomics. 2018;50(4):287–95. https://doi.org/10.1152/physiolgenomics.00090.2017.
Li, Guasch-Ferré, Chung, Ruiz-Canela, Toledo, Corella, et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J. 2020;41(28):2645–56. https://doi.org/10.1093/eurheartj/ehaa209.
Lima, Di Mascio, Rubbo, Abdalla. Characterization of linoleic acid nitration in human blood plasma by mass spectrometry. Biochemistry. 2002;41(34):10717–10722. https://doi.org/10.1021/bi025504j.
Lin, Zhu, Yang. Gut and obesity/metabolic disease: focus on microbiota metabolites. Med Comm (2020). 2022;3(3):e171. https://doi.org/10.1002/mco2.171.
Liu, Jia, Liu, Downton, Liu, Du, et al. Combined losartan and nitro-oleic acid remarkably improves diabetic nephropathy in mice. Am J Physiol Renal Physiol. 2013a;305(11):F1555–62. https://doi.org/10.1152/ajprenal.00157.2013.
Liu, Jia, Zhou, Liu, Ling, Zhou, et al.Nitro-oleic acid protects against adriamycin-induced nephropathy in mice. Am J Physiol Renal Physiol. 2013b;305(11):F1533–41. https://doi.org/10.1152/ajprenal.00656.2012.
Lonardo, Nascimbeni, Mantovani, Targher. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence?. J Hepatol. 2018;68(2):335–352. https://doi.org/10.1016/j.jhep.2017.09.021.
Lu, Sun, Liang, Zhang, Rom, Garcia-Barrio, et al. Novel gene regulatory networks identified in response to nitro-conjugated linoleic acid in human endothelial cells. Physiol Genomics. 2019;51(6):224–33. https://doi.org/10.1152/physiolgenomics.00127.2018.
Lundberg, Carlström, Weitzberg. Metabolic effects of dietary nitrate in health and disease. Cell Metab. 2018;28(1):9–22. https://doi.org/10.1016/j.cmet.2018.06.007.
Martin, Esser, Weber, Jakob, Freudenberg, Schmidt, et al. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy. 2011;66(9):1152–63. https://doi.org/10.1111/j.1398-9995.2011.02652.x.
Mastrogiovanni, Trostchansky, Rubbo. Data of detection and characterization of nitrated conjugated-linoleic acid (NO(2)-cLA) in LDL. Data Brief. 2020;28:105037. https://doi.org/10.1016/j.dib.2019.105037.
Mathers, Carey, Killeen, Diaz-Perez, Salvatore, Schopfer, et al. Electrophilic nitro-fatty acids suppress allergic contact dermatitis in mice. Allergy. 2017;72(4):656–64. https://doi.org/10.1111/all.13067.
McAlpine, Swirski. Circadian influence on metabolism and inflammation in atherosclerosis. Circ Res. 2016;119(1):131–141. https://doi.org/10.1161/circresaha.116.308034.
McGowan, Roumie. Sulfonylureas as second line treatment for type 2 diabetes. BMJ. 2018;362:k3041. https://doi.org/10.1136/bmj.k3041.
Mehta, Shapiro. Apolipoproteins in vascular biology and atherosclerotic disease. Nat Rev Cardiol. 2022;19(3):168–179. https://doi.org/10.1038/s41569-021-00613-5.
Melo, Montero-Bullón, Domingues, Domingues. Discovery of bioactive nitrated lipids and nitro-lipid-protein adducts using mass spectrometry-based approaches. Redox Biol. 2019;23:101106. https://doi.org/10.1016/j.redox.2019.101106.
Milewicz, Ramirez. Therapies for thoracic aortic aneurysms and acute aortic dissections. Arterioscler Thromb Vasc Biol. 2019;39(2):126–136. https://doi.org/10.1161/atvbaha.118.310956.
Milewicz, Braverman, De Backer, Morris, Boileau, Maumenee, et al. Marfan syndrome. Nat Rev Dis Primers. 2021;7(1):64. https://doi.org/10.1038/s41572-021-00298-7.
Milic, Griesser, Vemula, Ieda, Nakagawa, Miyata, et al. Profiling and relative quantification of multiply nitrated and oxidized fatty acids. Anal Bioanal Chem. 2015.407(19):5587–602. https://doi.org/10.1007/s00216-015-8766-3.
Mollenhauer M, Mehrkens D, Rudolph V. Nitrated fatty acids in cardiovascular diseases. Nitric Oxide. 2018:S1089–8603(17)30292-6. https://doi.org/10.1016/j.niox.2018.03.016.
Mollenhauer, Mehrkens, Klinke, Lange, Remane, Friedrichs, et al. Nitro-fatty acids suppress ischemic ventricular arrhythmias by preserving calcium homeostasis. Sci Rep. 2020;10(1):15319. https://doi.org/10.1038/s41598-020-71870-6.
Moore, Tabas. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. https://doi.org/10.1016/j.cell.2011.04.005.
Morigny, Boucher, Arner, Langin. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat Rev Endocrinol. 2021;17(5):276–295. https://doi.org/10.1038/s41574-021-00471-8.
Nadtochiy, Baker, Freeman, Brookes. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82(2):333–340. https://doi.org/10.1093/cvr/cvn323.
Narala, Thimmana, Panati, Kolliputi. Nitrated fatty acid, 10-nitrooleate protects against hyperoxia-induced acute lung injury in mice. Int Immunopharmacol. 2022;109:108838. https://doi.org/10.1016/j.intimp.2022.108838.
Nettersheim, Lemties, Braumann, Geißen, Bokredenghel, Nies, et al. Nitro-oleic acid reduces thoracic aortic aneurysm progression in a mouse model of Marfan syndrome. Cardiovasc Res. 2022;118(9):2211–25. https://doi.org/10.1093/cvr/cvab256.
Oduro, Zheng, Wei, Yang, Wang, Zhang, et al. The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy. Acta Pharm Sin B. 2022;12(1):50–75. https://doi.org/10.1016/j.apsb.2021.05.011.
Palmer, Strippoli. Metformin as first-line treatment for type 2 diabetes. Lancet. 2018;392(10142):120. https://doi.org/10.1016/s0140-6736(18)31541-1.
Pan, Xue, Auerbach, Fan, Bashore, Cui, et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation. 2020;142(21):2060–75. https://doi.org/10.1161/circulationaha.120.048378.
Pandey, Khan, Patel, Bhatt, Verma. Predicting and preventing heart failure in type 2 diabetes. Lancet Diabetes Endocrinol. 2023;11(8):607–624. https://doi.org/10.1016/s2213-8587(23)00128-6.
Piesche, Roos, Kühn, Fettel, Hellmuth, Brat, Maucher, et al. The emerging therapeutic potential of nitro fatty acids and other michael acceptor-containing drugs for the treatment of inflammation and cancer. Front Pharmacol. 2020;11:1297. https://doi.org/10.3389/fphar.2020.01297.
Pirruccello, Chaffin, Chou, Fleming, Lin, Nekoui, Khurshid, et al. Deep learning enables genetic analysis of the human thoracic aorta. Nat Genet. 2022;54(1):40–51. https://doi.org/10.1038/s41588-021-00962-4.
Poudyal, Brown. Should the pharmacological actions of dietary fatty acids in cardiometabolic disorders be classified based on biological or chemical function?. Prog Lipid Res. 2015;59:172–200. https://doi.org/10.1016/j.plipres.2015.07.002.
Qin, Wang. Protective roles of inorganic nitrate in health and diseases. Curr Med. 2022;1(1):4. https://doi.org/10.1007/s44194-022-00002-1.
Qipshidze-Kelm, Piell, Solinger, Cole. Co-treatment with conjugated linoleic acid and nitrite protects against myocardial infarction. Redox Biol. 2013;21–7. https://doi.org/10.1016/j.redox.2013.10.009.
Qiu, Abis, Mattingly-Peck, Lynham, Fraternali, Conte. Allosteric regulation of the soluble epoxide hydrolase by nitro fatty acids: a combined experimental and computational approach. J Mol Biol. 2022;434(17):167600. https://doi.org/10.1016/j.jmb.2022.167600.
Radi. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci U S A. 2018;115(23):5839–5848. https://doi.org/10.1073/pnas.1804932115.
Reddy, Lakshmi, Muchumarri, Reddy. Nitrated fatty acids reverse cigarette smoke-induced alveolar macrophage activation and inhibit protease activity via Electrophilic S-alkylation. PLoS One. 2016;11(4):e0153336. https://doi.org/10.1371/journal.pone.0153336.
Rocha, Gago, Barbosa, Lundberg, Radi, Laran**ha. Intragastric nitration by dietary nitrite: implications for modulation of protein and lipid signaling. Free Radic Biol Med. 2012;52(3):693–698. https://doi.org/10.1016/j.freeradbiomed.2011.11.011.
Rockey, Bell, Hill. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–1149. https://doi.org/10.1056/NEJMra1300575.
Rodriguez, Lavie, Elagizi, Milani. Update on Omega-3 polyunsaturated fatty acids on cardiovascular health. Nutrients. 2022;14(23). https://doi.org/10.3390/nu14235146.
Rodriguez-Duarte, Galliussi, Dapueto, Rossello, Malacrida, Kamaid, et al. A novel nitroalkene-alpha-tocopherol analogue inhibits inflammation and ameliorates atherosclerosis in Apo E knockout mice. Br J Pharmacol. 2019;176(6):757–72. https://doi.org/10.1111/bph.14561.
Rom, Xu, Guo, Zhu, Wang, Zhang,et al. Nitro-fatty acids protect against steatosis and fibrosis during development of nonalcoholic fatty liver disease in mice. EBioMedicine. 2019;4162–72. https://doi.org/10.1016/j.ebiom.2019.02.019.
Rom, Liu, Chang, Chen, Aviram. Editorial: nitro-fatty acids: novel drug candidates for the co-treatment of atherosclerosis and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2020;31(2):104–107. https://doi.org/10.1097/mol.0000000000000666.
Ros. Eat nuts, live longer.J Am Coll Cardiol. 2017;70(20):2533–2535. https://doi.org/10.1016/j.jacc.2017.09.1082.
Rosenblat, Rom, Volkova, Aviram. Nitro-oleic acid reduces J774A.1 macrophage oxidative status and triglyceride mass: involvement of paraoxonase2 and triglyceride metabolizing enzymes. Lipids. 2016;51(8):941–953. https://doi.org/10.1007/s11745-016-4169-2.
Rudolph, Schopfer, Khoo, Rudolph, Cole, Woodcock, et al. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem. 2009;284(3):1461–73. https://doi.org/10.1074/jbc.M802298200.
Rudolph, Rudolph, Edreira, Cole, Bonacci, Schopfer, et al. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010a;30(5):938–45. https://doi.org/10.1161/atvbaha.109.201582.
Rudolph, Rudolph, Schopfer, Bonacci, Woodcock, Cole, et al. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res. 2010b;85(1):155–66. https://doi.org/10.1093/cvr/cvp275.
Rudolph, Ravekes, Klinke, Friedrichs, Mollenhauer, Pekarova, et al. Nitrated fatty acids suppress angiotensin II-mediated fibrotic remodelling and atrial fibrillation. Cardiovasc Res. 2016;109(1):174–84. https://doi.org/10.1093/cvr/cvv254.
Rundblad, Sandoval, Holven, Ordovás, Ulven. Omega-3 fatty acids and individual variability in plasma triglyceride response: a mini-review. Redox Biol. 2023;63:102730. https://doi.org/10.1016/j.redox.2023.102730.
Sakalihasan, Michel, Katsargyris, Kuivaniemi, Defraigne, Nchimi, et al. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4(1):34. https://doi.org/10.1038/s41572-018-0030-7.
Salvatore, Vitturi, Baker, Bonacci, Koenitzer, Woodcock, et al. Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. J Lipid Res. 2013;54(7):1998–2009. https://doi.org/10.1194/jlr.M037804.
Salvatore, Rowart, Schopfer. Mass spectrometry-based study defines the human urine nitrolipidome. Free Radic Biol Med. 2021;162:327–337. https://doi.org/10.1016/j.freeradbiomed.2020.10.305.
Sánchez-Calvo, Cassina, Mastrogiovanni, Santos, Trias, Kelley, et al. Olive oil-derived nitro-fatty acids: protection of mitochondrial function in non-alcoholic fatty liver disease. J Nutr Biochem. 2021;94:108646.https://doi.org/10.1016/j.jnutbio.2021.108646
Schopfer, Lin, Baker, Cui, Garcia-Barrio, Zhang, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102(7):2340–5. https://doi.org/10.1073/pnas.0408384102.
Schopfer, Cole, Groeger, Chen, Khoo, Woodcock, et al. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem. 2010;285(16):12321–33. https://doi.org/10.1074/jbc.M109.091512.
Schopfer, Freeman, Khoo.Nitro-oleic acid and epoxyoleic acid are not altered in obesity and type 2 diabetes: reply. Cardiovasc Res. 2014;102(3):518. https://doi.org/10.1093/cvr/cvu042.
Schopfer, Vitturi, Jorkasky, Freeman. Nitro-fatty acids: New drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide. 2018;7931–7. https://doi.org/10.1016/j.niox.2018.06.006.
Sedeek, Callera, Montezano, Gutsol, Heitz, Szyndralewiez, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299(6):F1348–58. https://doi.org/10.1152/ajprenal.00028.2010.
Sherratt, Libby, Budoff, Bhatt, Mason. Role of omega-3 fatty acids in cardiovascular disease: the debate continues. Curr Atheroscler Rep. 2023;25(1):1–17. https://doi.org/10.1007/s11883-022-01075-x.
Shoaie, Ghaffari, Kovatcheva-Datchary, Mardinoglu, Sen, Pujos-Guillot, et al. Quantifying diet-induced metabolic changes of the human gut microbiome. Cell Metab. 2015;22(2):320–31. https://doi.org/10.1016/j.cmet.2015.07.001.
Shores, Berger, Murphy, Pyeritz. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330(19):1335–1341. https://doi.org/10.1056/nejm199405123301902.
Shu, Peng, Hang, Nie, Zhou, Wang. The role of CD36 in cardiovascular disease. Cardiovasc Res. 2022;118(1):115–129. https://doi.org/10.1093/cvr/cvaa319.
Stitziel, Stirrups, Masca, Erdmann, Ferrario, König, et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374(12):1134–44. https://doi.org/10.1056/NEJMoa1507652.
Tashiro, Koyanagi, Ohara, Ito, Saitoh, Horikoshi, et al. Levels of urinary matrix metalloproteinase-9 (MMP-9) and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2004;18(3):206–10. https://doi.org/10.1002/jcla.20024.
Thomas, Brownlee, Susztak, Sharma, Jandeleit-Dahm, Zoungas, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. https://doi.org/10.1038/nrdp.2015.18.
Tsikas, Batkai, Mitschke, Jordan, Engeli. Nitro-oleic acid and epoxy-oleic acid are not altered in obesity and type 2 diabetes. Cardiovasc Res. 2014;102(3):517–518. https://doi.org/10.1093/cvr/cvu043.
Tsikas. Measurement of nitro-oleic acid and nitro-linoleic acid in plasma by GC-MS/MS and LC-MS/MS in health and disease: the significance of the internal standard. J Chromatogr B Analyt Technol Biomed Life Sci. 2023;1221:123684.https://doi.org/10.1016/j.jchromb.2023.123684
Vazquez, Gutierrez, Salvatore, Puiatti, Dato, Chiabrando, et al. Nitro-oleic acid, a ligand of CD36, reduces cholesterol accumulation by modulating oxidized-LDL uptake and cholesterol efflux in RAW264.7 macrophages. Redox Biol. 2020;36:101591. https://doi.org/10.1016/j.redox.2020.101591.
Villacorta, Zhang, Garcia-Barrio, Chen, Freeman, Chen, et al. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293(1):H770–6. https://doi.org/10.1152/ajpheart.00261.2007.
Villacorta, Chang, Salvatore, Ichikawa, Zhang, Petrovic-Djergovic, et al. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res. 2013;98(1):116–24. https://doi.org/10.1093/cvr/cvt002.
Villacorta, Minarrieta, Salvatore, Khoo, Rom, Gao, et al. In situ generation, metabolism and immunomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox Biol. 2018;15:522–31. https://doi.org/10.1016/j.redox.2018.01.005.
Visseren, Mach, Smulders, Carballo, Koskinas, Bäck, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337. https://doi.org/10.1093/eurheartj/ehab484.
Vitturi, Chen, Woodcock, Salvatore, Bonacci, Koenitzer, et al. Modulation of nitro-fatty acid signaling: prostaglandin reductase-1 is a nitroalkene reductase. J Biol Chem. 2013;288(35):25626–37. https://doi.org/10.1074/jbc.M113.486282.
Vitturi, Maynard, Olsufka, Straub, Krehel, Kudenchuk,et al. Nitrite elicits divergent NO-dependent signaling that associates with outcome in out of hospital cardiac arrest. Redox Biol. 2020;32:101463. https://doi.org/10.1016/j.redox.2020.101463.
Waks, Winer. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. https://doi.org/10.1001/jama.2018.19323.
Wang, Hoyte. Review of Biguanide (Metformin) toxicity. J Intensive Care Med. 2019;34(11–12):863–876. https://doi.org/10.1177/0885066618793385.
Wang, Liu, Jia, Guan, Yang. Effects of endogenous PPAR agonist nitro-oleic acid on metabolic syndrome in obese zucker rats. PPAR Res. 2010;2010:601562.https://doi.org/10.1155/2010/601562.
Wang, Li, Yang. Protection of nitro-fatty acid against kidney diseases. Am J Physiol Renal Physiol. 2016;310(8):F697–704. https://doi.org/10.1152/ajprenal.00321.2015.
Wang, Wang, Antony, Sun, Liang. Metabolism-Associated Molecular Patterns (MAMPs). Trends Endocrinol Metab. 2020;31(10):712–724. https://doi.org/10.1016/j.tem.2020.07.001.
Wang, Killeen, Sumpter, Ferris, Falo, Freeman, et al. Electrophilic nitro-fatty acids suppress psoriasiform dermatitis: STAT3 inhibition as a contributory mechanism. Redox Biol. 2021;43:101987. https://doi.org/10.1016/j.redox.2021.101987.
Weinberg, Brook, Rubenfire, Eagle. Cardiovascular impact of nutritional supplementation with omega-3 fatty acids: JACC focus seminar. J Am Coll Cardiol. 2021;77(5):593–608. https://doi.org/10.1016/j.jacc.2020.11.060.
Wilkinson, Abramova, Guo, Gow, Murray, Koudelka, et al. Fatty acid nitroalkenes inhibit the inflammatory response to bleomycin-mediated lung injury. Toxicol Appl Pharmacol. 2020;407:115236. https://doi.org/10.1016/j.taap.2020.115236.
Wit, Trujillo-Viera, Strohmeyer, Klingenspor, Hankir, Sumara. When fat meets the gut-focus on intestinal lipid handling in metabolic health and disease. EMBO Mol Med. 2022;14(5):e14742. https://doi.org/10.15252/emmm.202114742.
Woodcock, Huang, Woodcock, Salvatore, Singh, Golin-Bisello,et al. Nitro-fatty acid inhibition of triple-negative breast cancer cell viability, migration, invasion, and tumor growth. J Biol Chem. 2018;293(4):1120–37. https://doi.org/10.1074/jbc.M117.814368.
Wright, Schopfer, Baker, Vidyasagar, Powell, Chumley, et al. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci U S A. 2006;103(11):4299–304. https://doi.org/10.1073/pnas.0506541103.
Wroński, Gęgotek, Skrzydlewska. Protein adducts with lipid peroxidation products in patients with psoriasis. Redox Biol. 2023;63:102729. https://doi.org/10.1016/j.redox.2023.102729.
Xourafa, Korbmacher, Roden. Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat Rev Endocrinol. 2024;20(1):27–49. https://doi.org/10.1038/s41574-023-00898-1.
Xu, Chuang, Hawkins, Hägglund, Davies. Identification and quantification of protein nitration sites in human coronary artery smooth muscle cells in the absence and presence of peroxynitrous acid/peroxynitrite. Redox Biol. 2023;64:102799. https://doi.org/10.1016/j.redox.2023.102799
Yang, Kyle, Makielski, Dudley. Mechanisms of sudden cardiac death: oxidants and metabolism. Circ Res. 2015;116(12):1937–1955. https://doi.org/10.1161/circresaha.116.304691.
Younossi, Anstee, Marietti, Hardy, Henry, Eslam,et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. https://doi.org/10.1038/nrgastro.2017.109.
Yu, Ramasamy, Fazzari, Chen, Freeman, Pacella. Lipid nitroalkene nanoparticles for the focal treatment of ischemia reperfusion. Nanotheranostics. 2022;6(2):215–229. https://doi.org/10.7150/ntno.62351.
Zatloukalova, Mojovic, Pavicevic, Kabelac, Freeman, et al. Redox properties and human serum albumin binding of nitro-oleic acid. Redox Biol. 2019;24:101213. https://doi.org/10.1016/j.redox.2019.101213.
Zeng, Luo, Wang, Zhang, Peng, Fan.Therapeutic Effect of curcumin on metabolic diseases: evidence from clinical studies. Int J Mol Sci. 2023;24(4). https://doi.org/10.3390/ijms24043323.
Zhang, Villacorta, Chang, Fan, Hamblin, Zhu, et al. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res. 2010;107(4):540–8. https://doi.org/10.1161/circresaha.110.218404.
Zhang, Liu, Duan, Li, Peng, Wu. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J Adv Res. 2021;34:43–63. https://doi.org/10.1016/j.jare.2021.06.023.
Zhao, Chang, Zhao, Lu, **ong, Liang, et al. Suppression of vascular macrophage activation by nitro-oleic acid and its implication for abdominal aortic aneurysm therapy. Cardiovasc Drugs Ther. 2021;35(5):939–51. https://doi.org/10.1007/s10557-020-07031-8.
Acknowledgements
This work was funded by the Bei**g Laboratory of Oral Health (PXM2021_014226_000041) and the National Key R&D Program of China (2021YFA0805100). Figures were created with BioRender.com.
Funding
Bei**g Laboratory of Oral Health (PXM2021_014226_000041) and the National Key R&D Program of China (2021YEA0805100).
Author information
Authors and Affiliations
Contributions
This review article was led and overseen by J.D. and Y.W, who provided guidance and supervision throughout the process. N.H. was responsible for planning and drafting the manuscript, based on the literature review and analysis. T.X. reviewed and enhanced the manuscript, ensuring its quality and accuracy. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
NA.
Consent for publication
All authors agree to publish.
Competing interests
The author Jie Du is a member of the Editorial Board for Current Medicine. The paper was handled by the other journal Editor and has undergone rigorous peer review process. Jie Du was not involved in the journal’s review of, or decisions related to, this manuscript. The other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ni, H., Tan, X., Du, J. et al. Nitro-fatty acids: mechanisms of action, roles in metabolic diseases, and therapeutics. Curr Med 3, 3 (2024). https://doi.org/10.1007/s44194-024-00030-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44194-024-00030-z